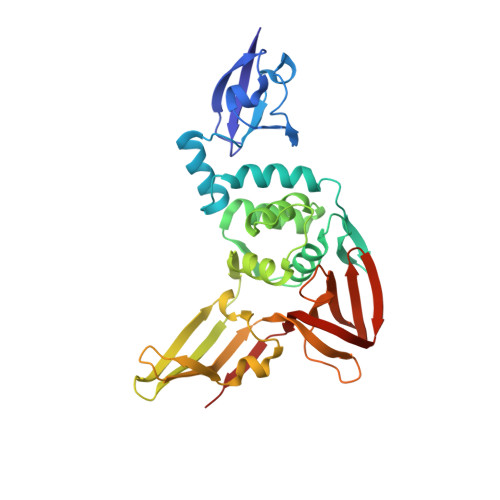
Structurally Guided Removal of DeISGylase Biochemical Activity from Papain-Like Protease Originating from Middle East Respiratory Syndrome Coronavirus.
Daczkowski, C.M., Goodwin, O.Y., Dzimianski, J.V., Farhat, J.J., Pegan, S.D.(2017) J Virol 91
- PubMed: 28931677
- DOI: https://doi.org/10.1128/JVI.01067-17
- PubMed Abstract:
Middle East respiratory syndrome coronavirus (MERS-CoV) is an emerging human pathogen that is the causative agent for Middle East respiratory syndrome (MERS). With MERS outbreaks resulting in over 35% fatalities and now spread to 27 countries, MERS-CoV poses a significant ongoing threat to global human health. As part of its viral genome, MERS-CoV encodes a papain-like protease (PLpro) that has been observed to act as a deubiquitinase and deISGylase to antagonize type I interferon (IFN-I) immune pathways. This activity is in addition to its viral polypeptide cleavage function. Although the overall impact of MERS-CoV PLpro function is observed to be essential, difficulty has been encountered in delineating the importance of its separate functions, particularly its deISGylase activity. As a result, the interface of MERS-CoV and human interferon-stimulated gene product 15 (hISG15) was probed with isothermal calorimetry, which suggests that the C-terminal domain of hISG15 is principally responsible for interactions. Subsequently, the structure of MERS-CoV PLpro was solved to 2.4 Å in complex with the C-terminal domain of hISG15. Utilizing this structural information, mutants were generated that lacked appreciable deISGylase activity but retained wild-type deubiquitinase and peptide cleavage activities. Hence, this provides a new platform for understanding viral deISGylase activity within MERS-CoV and other CoVs. IMPORTANCE Coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV), encode a papain-like protease (PLpro) that possesses the ability to antagonize interferon immune pathways through the removal of ubiquitin and interferon-stimulated gene product 15 (ISG15) from target proteins. The lack of CoV proteases with attenuated deISGylase activity has been a key obstacle in delineating the impact between deubiquitinase and deISGylase activities on viral host evasion and pathogenesis. Here, biophysical techniques revealed that MERS-CoV PLpro chiefly engages human ISG15 through its C-terminal domain. The first structure of MERS-CoV PLpro in complex with this domain exposed the interface between these two entities. Employing these structural insights, mutations were employed to selectively remove deISGylase activity with no appreciable impact on its other deubiquitinase and peptide cleavage biochemical properties. Excitingly, this study introduces a new tool to probe the pathogenesis of MERS-CoV and related viruses through the removal of viral deISGylase activity.
Organizational Affiliation:
Pharmaceutical and Biomedical Sciences Department, University of Georgia, Athens, Georgia, USA.