Crystal structure of a complex of phospholipase A2 with a peptide Ala-Ile-Ala-Ser at 2.8 A resolution
Kumar, S., Singh, N., Sharma, S., Kaur, P., Singh, T.P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phospholipase A2 VRV-PL-VIIIa | 121 | Daboia russelii pulchella | Mutation(s): 0 EC: 3.1.1.4 | 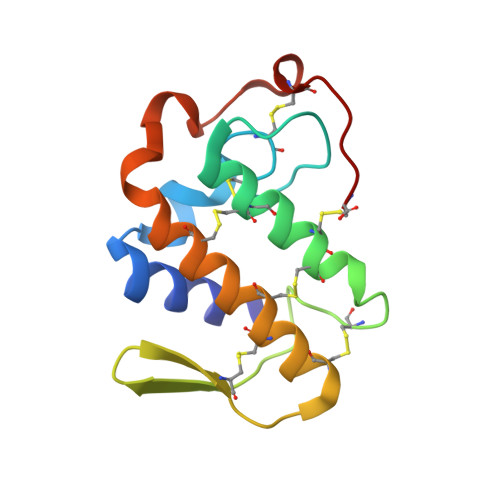 | |
UniProt | |||||
Find proteins for P59071 (Daboia russelii) Explore P59071 Go to UniProtKB: P59071 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P59071 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ala-Ile-Ala-Ser peptide | B [auth P] | 4 | N/A | Mutation(s): 0 | 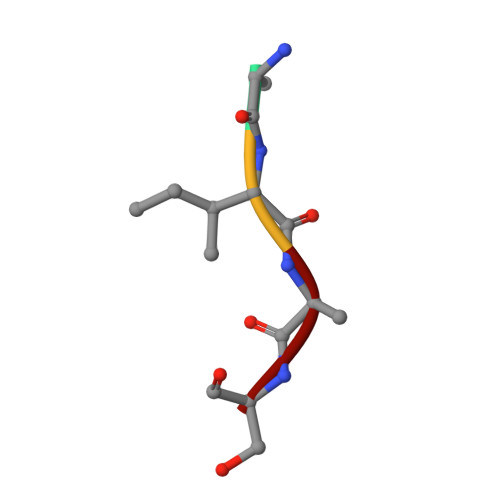 |
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 52.96 | α = 90 |
| b = 52.96 | β = 90 |
| c = 48.63 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| MAR345 | data collection |
| AUTOMAR | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |