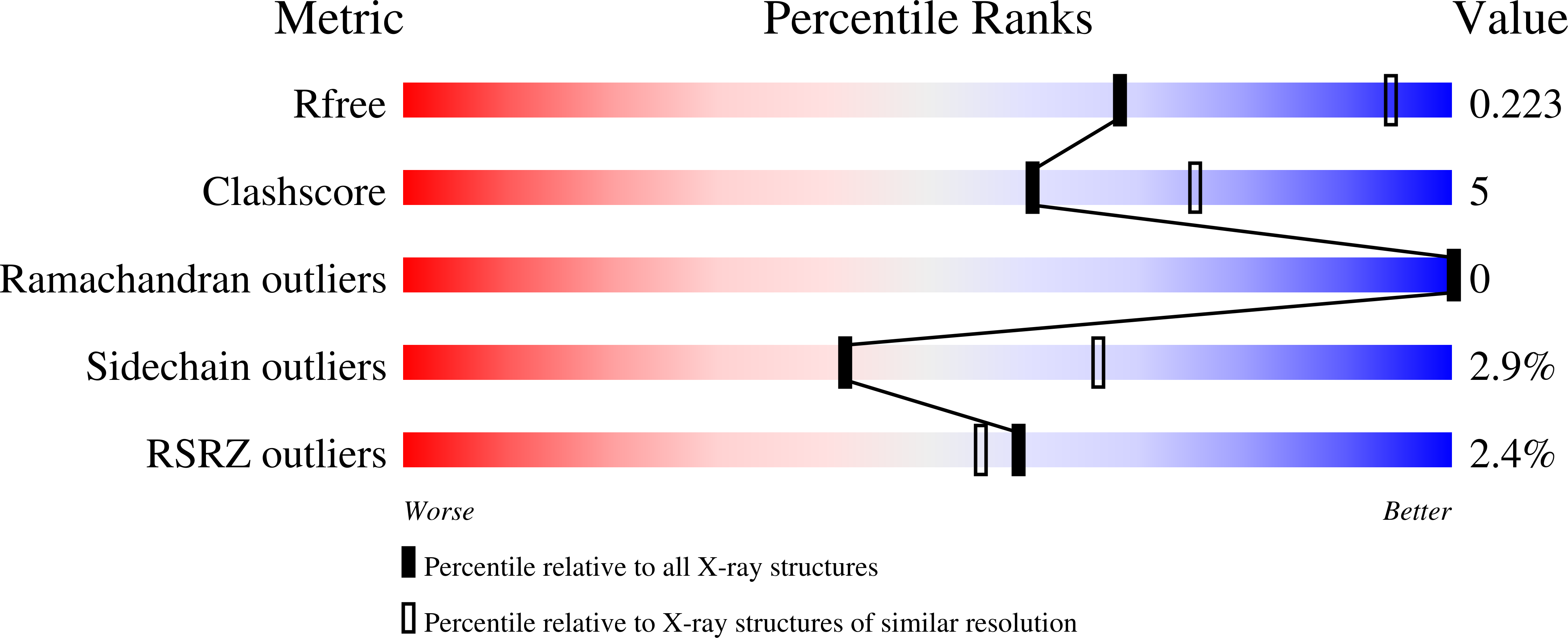
Structural evidence for a sequential release mechanism for activation of heterotrimeric g proteins.
Kapoor, N., Menon, S.T., Chauhan, R., Sachdev, P., Sakmar, T.P.(2009) J Mol Biol 393: 882-897
- PubMed: 19703466
- DOI: https://doi.org/10.1016/j.jmb.2009.08.043
- Primary Citation of Related Structures:
3FFA, 3FFB - PubMed Abstract:
Heptahelical G-protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors couple to heterotrimeric G proteins to relay extracellular signals to intracellular signaling networks, but the molecular mechanism underlying guanosine 5'-diphosphate (GDP) release by the G protein alpha-subunit is not well understood. Amino acid substitutions in the conserved alpha5 helix of G(i), which extends from the C-terminal region to the nucleotide-binding pocket, cause dramatic increases in basal (receptor-independent) GDP release rates. For example, mutant Galpha(i1)-T329A shows an 18-fold increase in basal GDP release rate and, when expressed in culture, it causes a significant decrease in forskolin-stimulated cAMP accumulation. The crystal structure of Galpha(i1)-T329A.GDP shows substantial conformational rearrangement of the switch I region and additional striking alterations of side chains lining the catalytic pocket that disrupt the Mg(+2) coordination sphere and dislodge bound Mg(+2). We propose a "sequential release" mechanism whereby a transient conformational change in the alpha5 helix alters switch I to induce GDP release. Interestingly, this mechanistic model for heterotrimeric G protein activation is similar to that suggested for the activation of the plant small G protein Rop4 by RopGEF8.
Organizational Affiliation:
Laboratory of Biochemistry and Molecular Biology, Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.