Active-Site Structure of Class IV Adenylyl Cyclase and Transphyletic Mechanism.
Gallagher, D.T., Kim, S.K., Robinson, H., Reddy, P.T.(2011) J Mol Biol 405: 787-803
- PubMed: 21094652
- DOI: https://doi.org/10.1016/j.jmb.2010.11.026
- Primary Citation of Related Structures:
3N0Y, 3N0Z, 3N10 - PubMed Abstract:
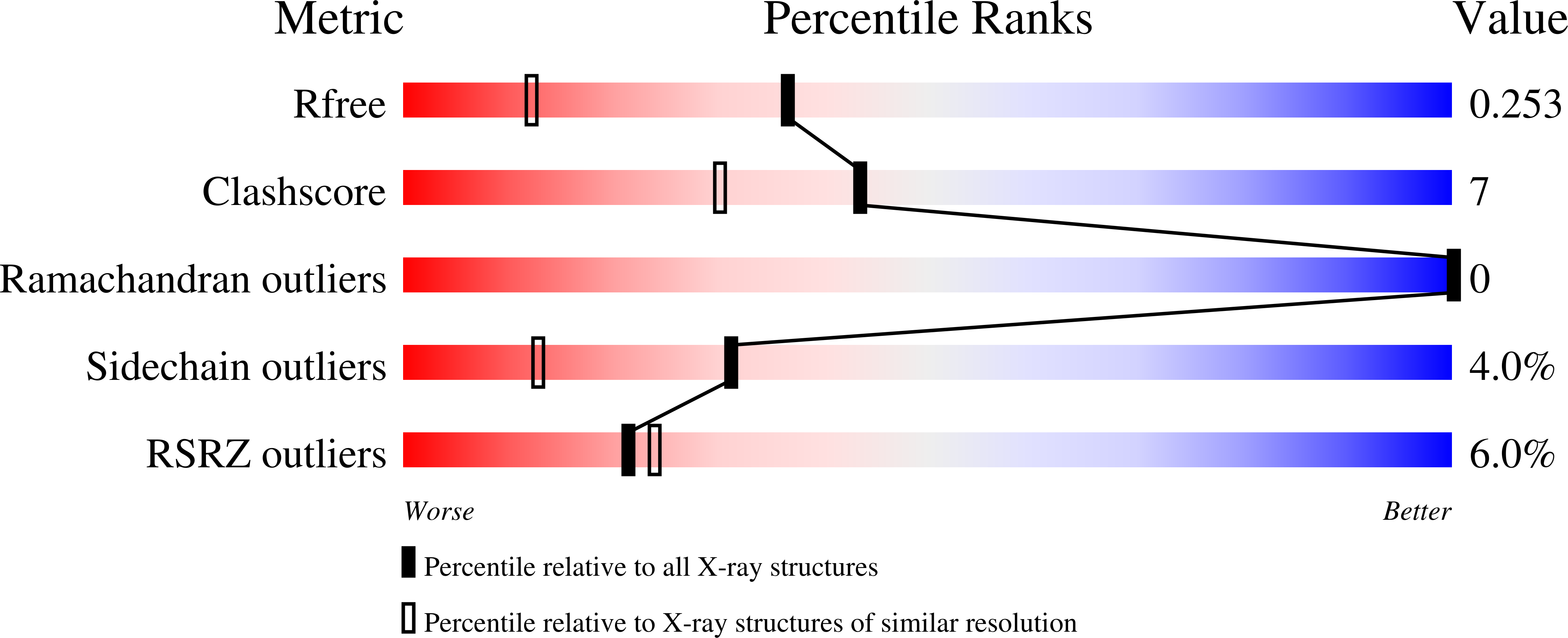
Adenylyl cyclases (ACs) belonging to three nonhomologous classes (II, III, and IV) have been structurally characterized, enabling a comparison of the mechanisms of cyclic adenosine 3',5'-monophosphate biosynthesis. We report the crystal structures of three active-site complexes for Yersinia pestis class IV AC (AC-IV)-two with substrate analogs and one with product. Mn(2+) binds to all three phosphates, and to Glu12 and Glu136. Electropositive residues Lys14, Arg63, Lys76, Lys111, and Arg113 also form hydrogen bonds to phosphates. The conformation of the analogs is suitable for in-line nucleophilic attack by the ribose O3' on α-phosphate (distance ∼4 Å). In the product complex, a second Mn ion is observed to be coordinated to both ribose 2' oxygen and ribose 3' oxygen. Observation of both metal sites, together with kinetic measurements, provides strong support for a two-cation mechanism. Eleven active-site mutants were also made and kinetically characterized. These findings and comparisons with class II and class III enzymes enable a detailed transphyletic analysis of the AC mechanism. Consistent with its lack of coordination to purine, Y. pestis AC-IV cyclizes both ATP and GTP. As in other classes of AC, the ribose is loosely bound, and as in class III, no base appears to ionize the O3' nucleophile. Different syn/anti conformations suggest that the mechanism involves a conformational transition, and further evidence suggests a role for ribosyl pseudorotation. With resolutions of 1.6-1.7 Å, these are the most detailed active-site ligand complexes for any class of this ubiquitous signaling enzyme.
Organizational Affiliation:
Biochemical Science Division, Chemical Science and Technology Laboratory, National Institute of Standards and Technology, Gaithersburg, MD 20899, USA. gallagher@ibbr.umd.edu