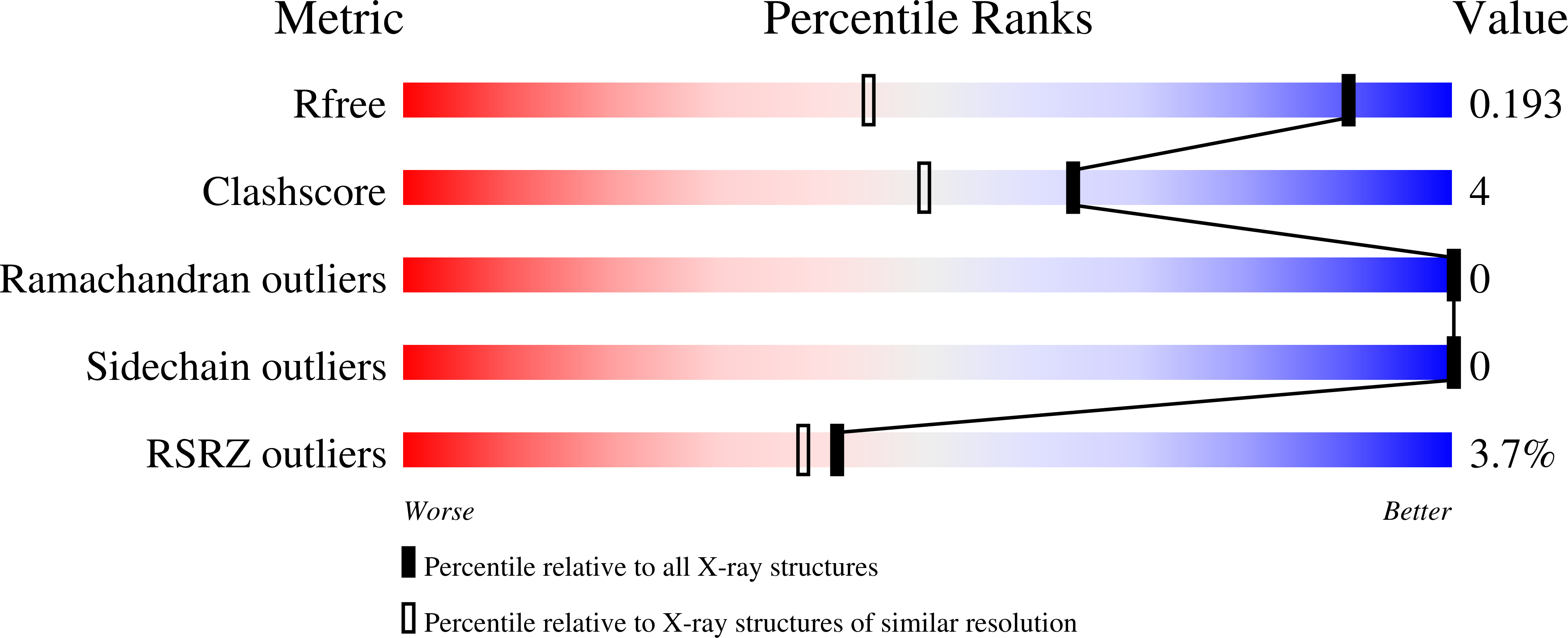
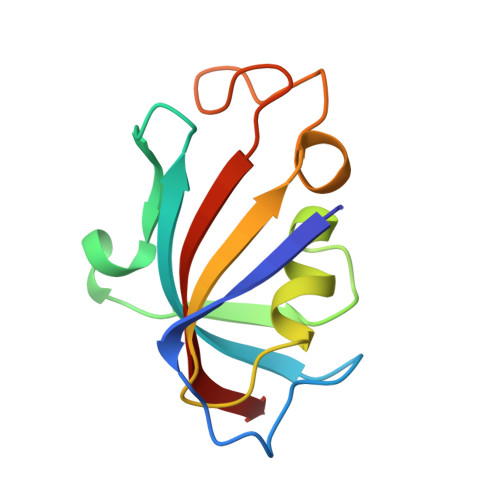
High-resolution crystal structure of FKBP12 from Aedes aegypti.
Rajan, S., Saw, K.Q., Nguyen, Q.T., Baek, K., Yoon, H.S.(2012) Protein Sci 21: 1080-1084
- PubMed: 22517662
- DOI: https://doi.org/10.1002/pro.2079
- Primary Citation of Related Structures:
3UQI - PubMed Abstract:
Dengue is one of the most infectious viral diseases prevalent mainly in tropical countries. The virus is transmitted by Aedes species of mosquito, primarily Aedes aegypti. Dengue remains a challenging drug target for years as the virus eludes the immune responses. Currently, no vaccines or antiviral drugs are available for dengue prevention. Previous studies suggested that the immunosuppressive drug FK506 shows antimalarial activity, and its molecular target, FK506-binding protein (FKBP), was identified in the Plasmodium parasite. Likewise, a FKBP family protein has been identified in A. aegypti (AaFKBP12) in which AaFKBP12 is assumed to play a similar role in its life cycle. FKBPs belong to a highly conserved class of proteins and are considered as an attractive pharmacological target. Herein, we present a high-resolution crystal structure of AaFKBP12 at 1.3 Å resolution and discuss its structural features throwing light in facilitating the design of potential antagonists against the dengue-transmitting mosquito.
Organizational Affiliation:
Division of Structural Biology and Biochemistry, School of Biological Science, Nanyang Technological University, Singapore 637551.