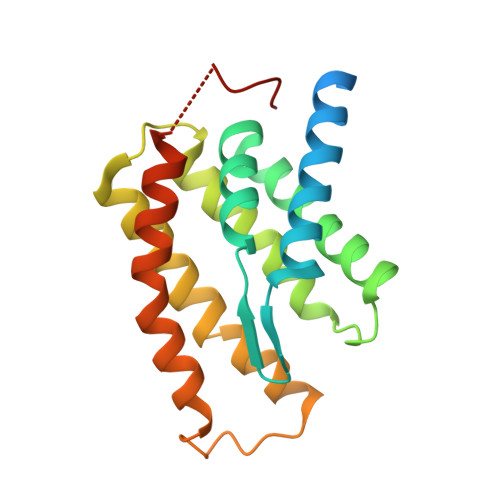
Structures of FolT in substrate-bound and substrate-released conformations reveal a gating mechanism for ECF transporters
Zhao, Q., Wang, C.C., Wang, C.Y., Guo, H., Bao, Z.H., Zhang, M.H., Zhang, P.(2015) Nat Commun 6: 7661-7661
- PubMed: 26198469
- DOI: https://doi.org/10.1038/ncomms8661
- Primary Citation of Related Structures:
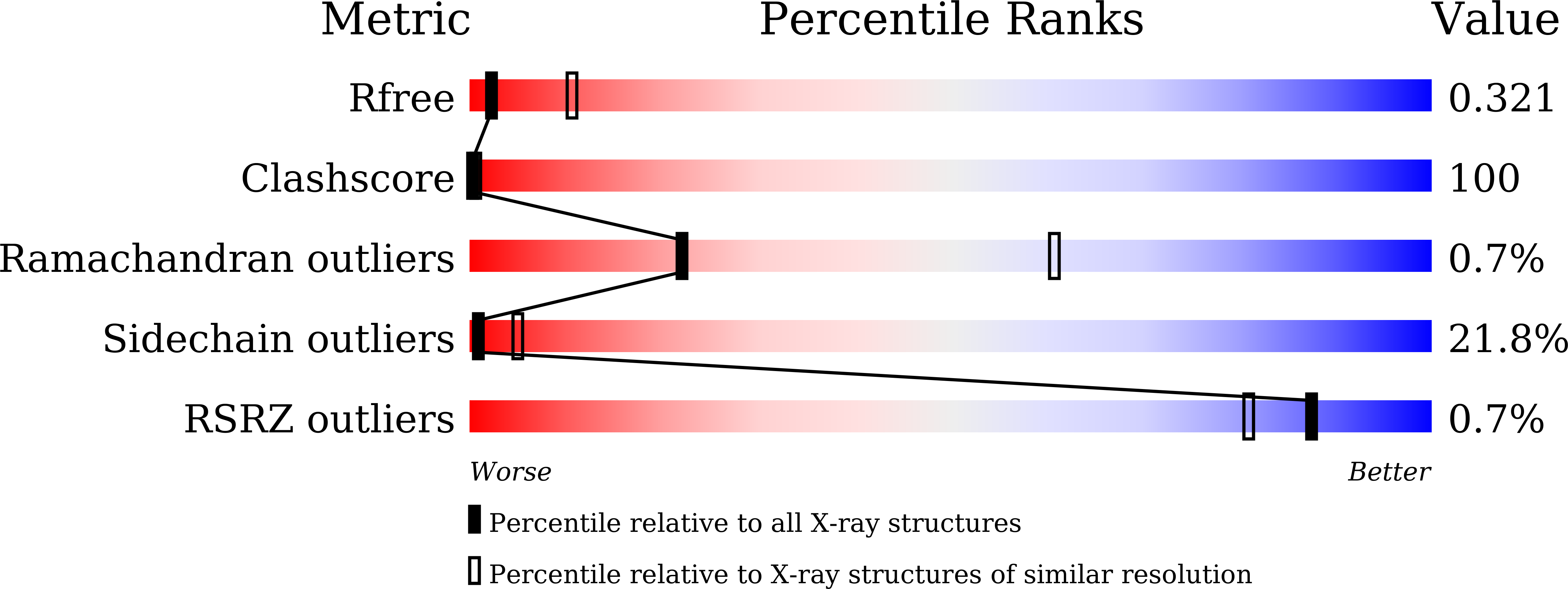
4Z7F - PubMed Abstract:
Energy-coupling factor (ECF) transporters are a new family of ABC transporters that consist of four subunits, two cytoplasmic ATPases EcfA and EcfA' and two transmembrane proteins namely EcfS for substrate-specific binding and EcfT for energy coupling. Here, we report the 3.2-Å resolution crystal structure of the EcfS protein of a folate ECF transporter from Enterococcus faecalis-EfFolT, a close homologue of FolT from Lactobacillus brevis-LbFolT. Structural and biochemical analyses reveal the residues constituting the folate-binding pocket and determining the substrate-binding specificity. Structural comparison of the folate-bound EfFolT with the folate-free LbFolT contained in the holotransporter complex discloses significant conformational change at the L1 loop, and reveals a gating mechanism of ECF transporters in which the L1 loop of EcfS acts as a gate in the substrate binding and release.
Organizational Affiliation:
National Key Laboratory of Plant Molecular Genetics, Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200032, China.