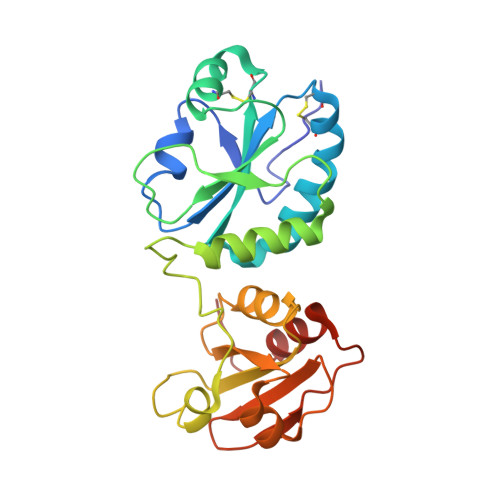
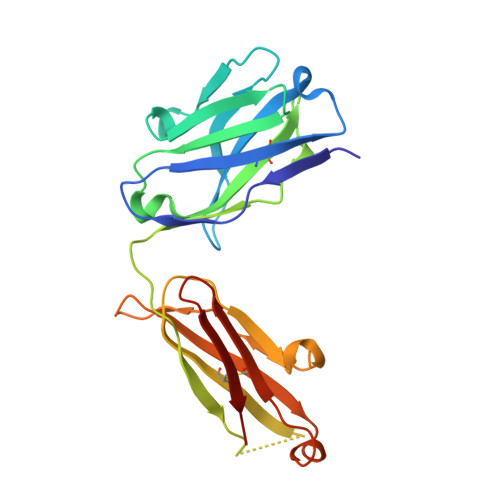
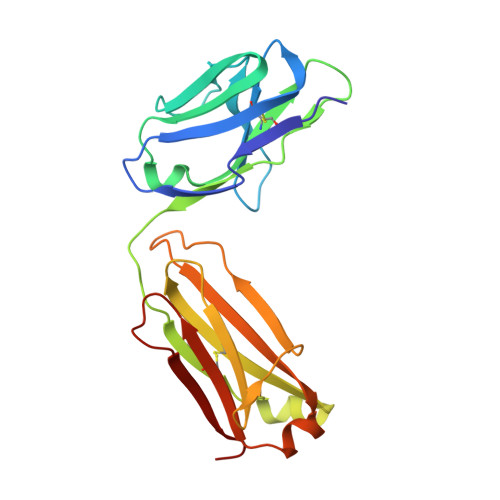
Overcoming a species-specificity barrier in development of an inhibitory antibody targeting a modulator of tumor stroma.
Grossman, I., Ilani, T., Fleishman, S.J., Fass, D.(2016) Protein Eng Des Sel 29: 135-147
- PubMed: 26819240
- DOI: https://doi.org/10.1093/protein/gzv067
- Primary Citation of Related Structures:
5D8I, 5D93, 5D96 - PubMed Abstract:
The secreted disulfide catalyst Quiescin sulfhydryl oxidase-1 (QSOX1) affects extracellular matrix organization and is overexpressed in various adenocarcinomas and associated stroma. Inhibition of extracellular human QSOX1 by a monoclonal antibody decreased tumor cell migration in a cell co-culture model and hence may have therapeutic potential. However, the species specificity of the QSOX1 monoclonal antibody has been a setback in assessing its utility as an anti-metastatic agent in vivo, a common problem in the antibody therapy industry. We therefore used structurally guided engineering to expand the antibody species specificity, improving its affinity toward mouse QSOX1 by at least four orders of magnitude. A crystal structure of the re-engineered variant, complexed with its mouse antigen, revealed that the antibody accomplishes dual-species targeting through altered contacts between its heavy and light chains, plus replacement of bulky aromatics by flexible side chains and versatile water-bridged polar interactions. In parallel, we produced a surrogate antibody targeting mouse QSOX1 that exhibits a new QSOX1 inhibition mode. This set of three QSOX1 inhibitory antibodies is compatible with various mouse models for pre-clinical trials and biotechnological applications. In this study we provide insights into structural blocks to cross-reactivity and set up guideposts for successful antibody design and re-engineering.
Organizational Affiliation:
Department of Structural Biology, Weizmann Institute of Science, Rehovot 7610001, Israel.