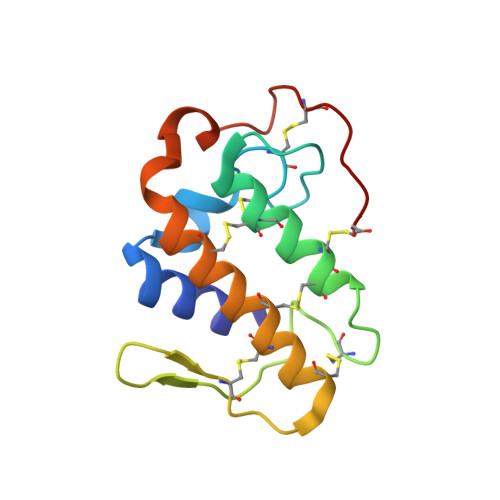
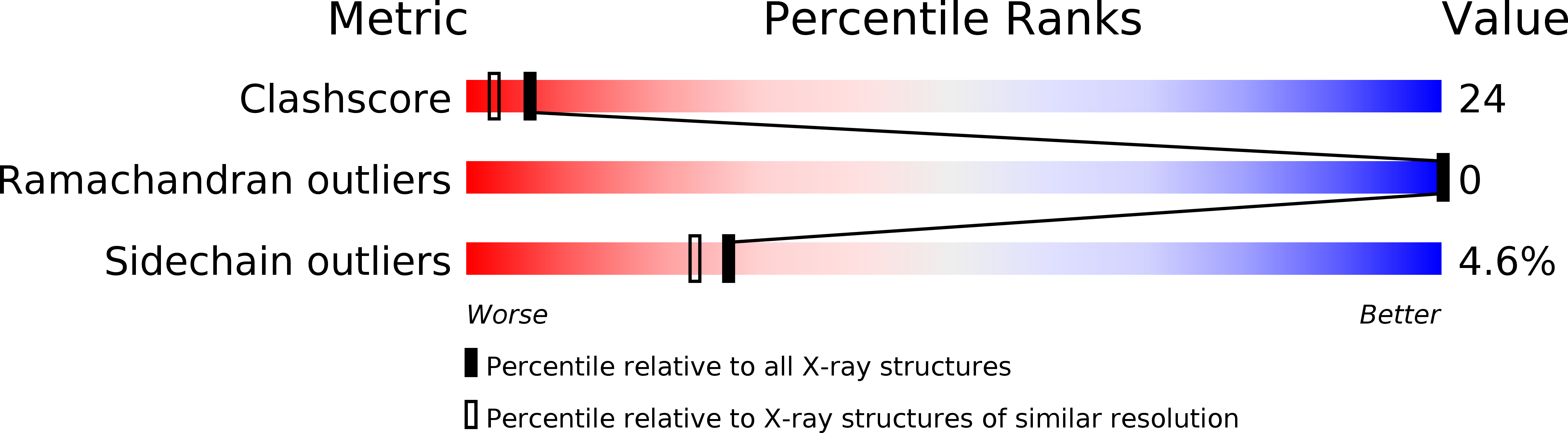The crystal structure of a lysine 49 phospholipase A2 from the venom of the cottonmouth snake at 2.0-A resolution.
Holland, D.R., Clancy, L.L., Muchmore, S.W., Ryde, T.J., Einspahr, H.M., Finzel, B.C., Heinrikson, R.L., Watenpaugh, K.D.(1990) J Biol Chem 265: 17649-17656
- PubMed: 2120215
- DOI: https://doi.org/10.2210/pdb1ppa/pdb
- Primary Citation of Related Structures:
1PPA - PubMed Abstract:
The crystal structure of a lysine 49 variant phospholipase A2 (K49 PLA2) has been determined at 2.0-A resolution. This particular phospholipase A2, purified from the venom of the eastern cottonmouth (Agkistrodon piscivorus piscivorus), a North American pit viper, differs significantly from others studied crystallographically because of replacement of the aspartate residue at position 49, whose side chain is important in calcium binding, by lysine. The crystallographic analysis of K49 PLA2 was undertaken to assess the structural ramifications of this substitution, particularly as they affect the binding mechanism of both the calcium cofactor and the phospholipid substrate. The protein crystals are tetragonal, space group P4(1)2(1)2, with unit cell dimensions of a = b = 71.7 (1) and c = 57.8 (3) A. Preliminary phases were obtained by molecular replacement techniques with a search model derived from the refined 2.5-A structure of a rattle-snake venom phospholipase A2 (Brunie, S., Bolin, J., Gewirth, D., and Sigler, P. B. (1985) J. Biol. Chem. 260, 9742-9749). The starting model gave an initial crystallographic RF of 0.526 (RF = sigma parallel to Fo /-/ Fc parallel to /sigma/Fo/). The structure was refined against all data to 2.0-A resolution. The final RF is 0.158. The final model includes 150 discrete water molecules. The K49 PLA2 model is composed primarily of alpha-helices joined by loops, some of which are quite extensive. Although dissimilarities are observed in the loop regions, the helical portions are very similar to those in other known phospholipase A2 structures. The proposed catalytic center (His48, Tyr73, and Asp99) is also structurally conserved. The region in K49 PLA2 corresponding to the calcium-binding site in other phospholipases A2 is occupied by the epsilon-amino group of lysine 49.
Organizational Affiliation:
Pharmaceutical Research and Development, Upjohn Company, Kalamazoo, Michigan 49007.