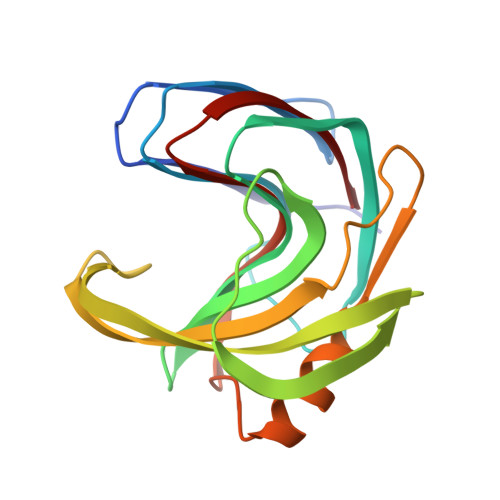
Correlation of temperature induced conformation change with optimum catalytic activity in the recombinant G/11 xylanase A from Bacillus subtilis strain 168 (1A1).
Murakami, M.T., Arni, R.K., Vieira, D.S., Degreve, L., Ruller, R., Ward, R.J.(2005) FEBS Lett 579: 6505-6510
- PubMed: 16289057
- DOI: https://doi.org/10.1016/j.febslet.2005.10.039
- Primary Citation of Related Structures:
1XXN - PubMed Abstract:
The 1.7A resolution crystal structure of recombinant family G/11 beta-1,4-xylanase (rXynA) from Bacillus subtilis 1A1 shows a jellyroll fold in which two curved beta-sheets form the active-site and substrate-binding cleft. The onset of thermal denaturation of rXynA occurs at 328 K, in excellent agreement with the optimum catalytic temperature. Molecular dynamics simulations at temperatures of 298-328 K demonstrate that below the optimum temperature the thumb loop and palm domain adopt a closed conformation. However, at 328 K these two domains separate facilitating substrate access to the active-site pocket, thereby accounting for the optimum catalytic temperature of the rXynA.
Organizational Affiliation:
Department of Physics, IBILCE/UNESP, Cristovão Colombo 2265, São José do Rio Preto, São Paulo, Brazil.