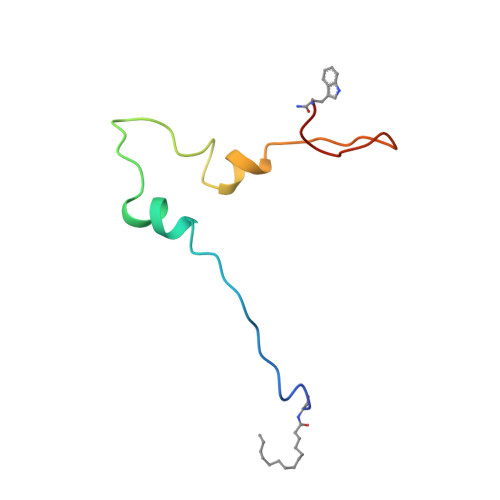
Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein.
Geyer, M., Munte, C.E., Schorr, J., Kellner, R., Kalbitzer, H.R.(1999) J Mol Biol 289: 123-138
- PubMed: 10339411
- DOI: https://doi.org/10.1006/jmbi.1999.2740
- Primary Citation of Related Structures:
1QA4, 1QA5 - PubMed Abstract:
Negative factor (Nef) is a regulatory myristoylated protein of human immunodeficiency virus (HIV) that has a two-domain structure consisting of an anchor domain and a core domain separated by a specific cleavage site of the HIV proteases. For structural analysis, the HIV-1 Nef anchor domain (residues 2-57) was synthesized with a myristoylated and non-myristoylated N terminus. The structures of the two peptides were studied by1H NMR spectroscopy and a structural model was obtained by restrained molecular dynamic simulations. The non-myristoylated peptide does not have a unique, compactly folded structure but occurs in a relatively extended conformation. The only rather well-defined canonical secondary structure element is a short two-turn alpha-helix (H2) between Arg35 and Gly41. A tendency for another helical secondary structure element (H1) can be observed for the arginine-rich region (Arg17 to Arg22). Myristoylation of the N-terminal glycine residue leads to stabilization of both helices, H1 and H2. The first helix in the arginine-rich region is stabilized by the myristoylation and now contains residues Pro14 to Arg22. The second helix appears to be better defined and to contain more residues (Ala33 to Gly41) than in the absence of myristoylation. In addition, the hydrophobic N-terminal myristic acid residue interacts closely with the side-chain of Trp5 and thereby forms a loop with Gly2, Gly3 and Lys4 in the kink region. This interaction could possibly be disturbed by phosphorylation of a nearby serine residue, and modifiy the characteristic membrane interactions of the HIV-1 Nef anchor domain.
Organizational Affiliation:
Abteilung Biophysik, Max-Planck-Institut für medizinische Forschung, Heidelberg, D-69120, Germany.