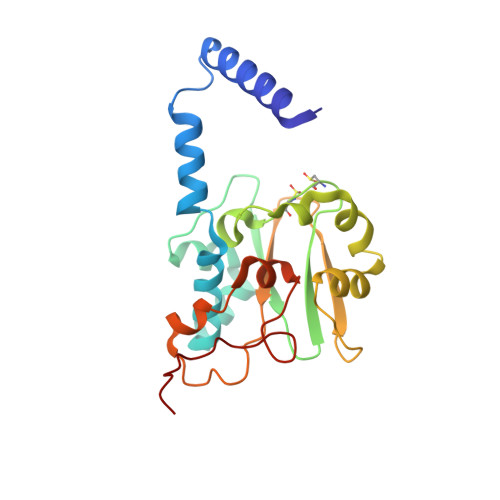
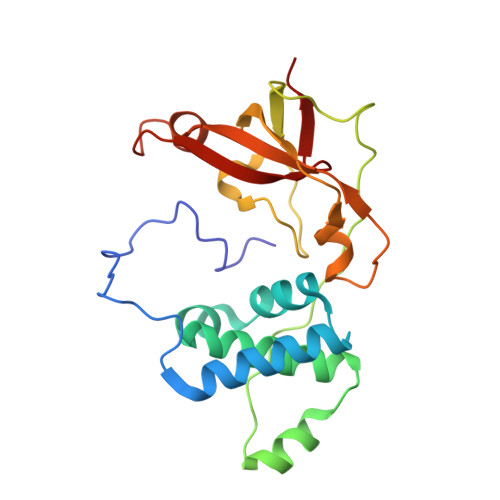
Analyzing the catalytic role of active site residues in the Fe-type nitrile hydratase from Comamonas testosteroni Ni1.
Martinez, S., Wu, R., Krzywda, K., Opalka, V., Chan, H., Liu, D., Holz, R.C.(2015) J Biol Inorg Chem 20: 885-894
- PubMed: 26077812
- DOI: https://doi.org/10.1007/s00775-015-1273-3
- Primary Citation of Related Structures:
4ZGD, 4ZGE, 4ZGJ - PubMed Abstract:
A strictly conserved active site arginine residue (αR157) and two histidine residues (αH80 and αH81) located near the active site of the Fe-type nitrile hydratase from Comamonas testosteroni Ni1 (CtNHase), were mutated. These mutant enzymes were examined for their ability to bind iron and hydrate acrylonitrile. For the αR157A mutant, the residual activity (k cat = 10 ± 2 s(-1)) accounts for less than 1% of the wild-type activity (k cat = 1100 ± 30 s(-1)) while the K m value is nearly unchanged at 205 ± 10 mM. On the other hand, mutation of the active site pocket αH80 and αH81 residues to alanine resulted in enzymes with k cat values of 220 ± 40 and 77 ± 13 s(-1), respectively, and K m values of 187 ± 11 and 179 ± 18 mM. The double mutant (αH80A/αH81A) was also prepared and provided an enzyme with a k cat value of 132 ± 3 s(-1) and a K m value of 213 ± 61 mM. These data indicate that all three residues are catalytically important, but not essential. X-ray crystal structures of the αH80A/αH81A, αH80W/αH81W, and αR157A mutant CtNHase enzymes were solved to 2.0, 2.8, and 2.5 Å resolutions, respectively. In each mutant enzyme, hydrogen-bonding interactions crucial for the catalytic function of the αCys(104)-SOH ligand are disrupted. Disruption of these hydrogen bonding interactions likely alters the nucleophilicity of the sulfenic acid oxygen and the Lewis acidity of the active site Fe(III) ion.
Organizational Affiliation:
Department of Chemistry, Marquette University, Milwaukee, WI, 53201, USA.