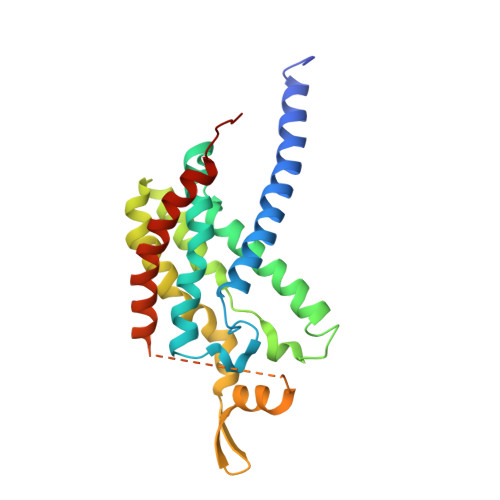
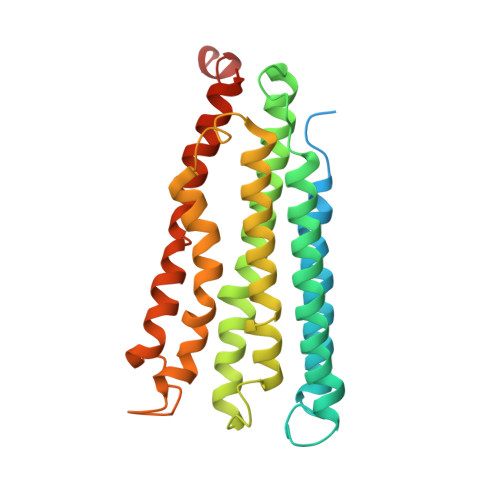
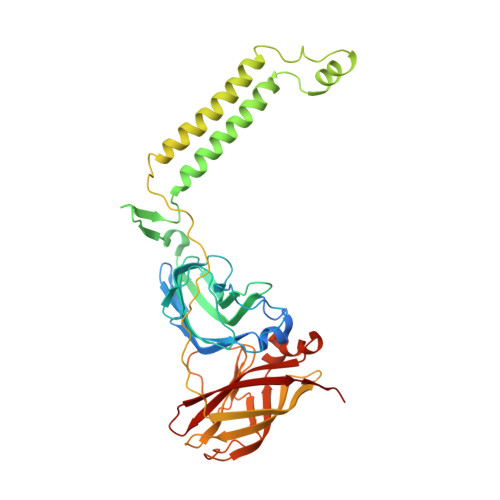
Structure and activity of particulate methane monooxygenase arrays in methanotrophs.
Zhu, Y., Koo, C.W., Cassidy, C.K., Spink, M.C., Ni, T., Zanetti-Domingues, L.C., Bateman, B., Martin-Fernandez, M.L., Shen, J., Sheng, Y., Song, Y., Yang, Z., Rosenzweig, A.C., Zhang, P.(2022) Nat Commun 13: 5221-5221
- PubMed: 36064719
- DOI: https://doi.org/10.1038/s41467-022-32752-9
- Primary Citation of Related Structures:
7YZY - PubMed Abstract:
Methane-oxidizing bacteria play a central role in greenhouse gas mitigation and have potential applications in biomanufacturing. Their primary metabolic enzyme, particulate methane monooxygenase (pMMO), is housed in copper-induced intracytoplasmic membranes (ICMs), of which the function and biogenesis are not known. We show by serial cryo-focused ion beam (cryoFIB) milling/scanning electron microscope (SEM) volume imaging and lamellae-based cellular cryo-electron tomography (cryoET) that these ICMs are derived from the inner cell membrane. The pMMO trimer, resolved by cryoET and subtomogram averaging to 4.8 Å in the ICM, forms higher-order hexagonal arrays in intact cells. Array formation correlates with increased enzymatic activity, highlighting the importance of studying the enzyme in its native environment. These findings also demonstrate the power of cryoET to structurally characterize native membrane enzymes in the cellular context.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK.