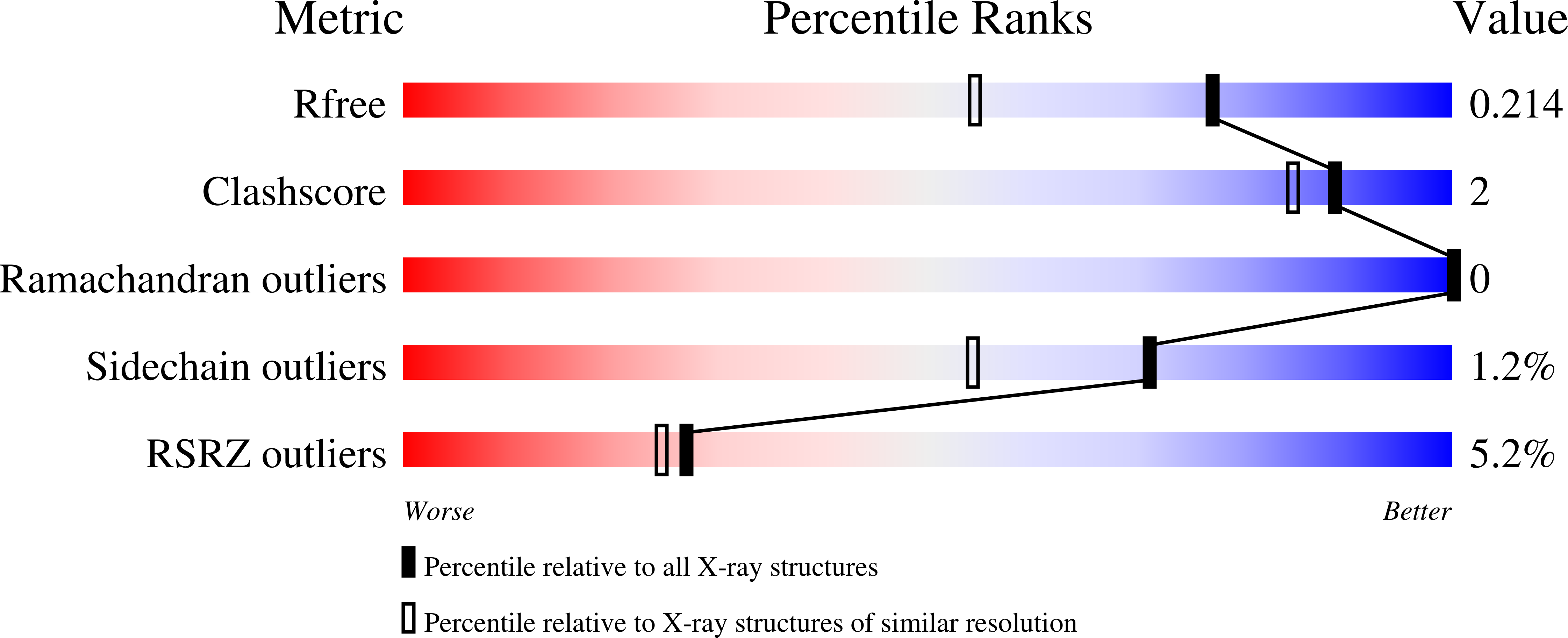
Crystallization and preliminary X-ray structure analysis of human ribosomal protein L30e
Kawaguchi, A., Ose, T., Yao, M., Tanaka, I.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 1516-1518
- PubMed: 22139155
- DOI: https://doi.org/10.1107/S1744309111045131
- Primary Citation of Related Structures:
3VI6 - PubMed Abstract:
Many functions have been reported for the eukaryotic ribosomal protein L30e. L30e makes several inter-subunit and intra-subunit interactions with protein or RNA components of the 80S ribosome. Yeast L30e has been shown to bind to its own transcript to autoregulate expression at both the transcriptional and the translational levels. Furthermore, it has been reported that mammalian L30e is a component of the selenocysteine-incorporation machinery by binding to the selenocysteine-insertion sequence on mRNA. As high-resolution crystal structures of mammalian L30e are not available, the purification, crystallization and X-ray structure analysis of human L30e are presented here.
Organizational Affiliation:
Graduate School of Life Sciences, Hokkaido University, Hokkaido, Sapporo, Japan.