2-Aminoadipic Acid-C(O)-Glutamate Based Prostate-Specific Membrane Antigen Ligands for Potential Use as Theranostics.
Nakajima, R., Novakova, Z., Tueckmantel, W., Motlova, L., Barinka, C., Kozikowski, A.P.(2018) ACS Med Chem Lett 9: 1099-1104
- PubMed: 30429952
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00318
- Primary Citation of Related Structures:
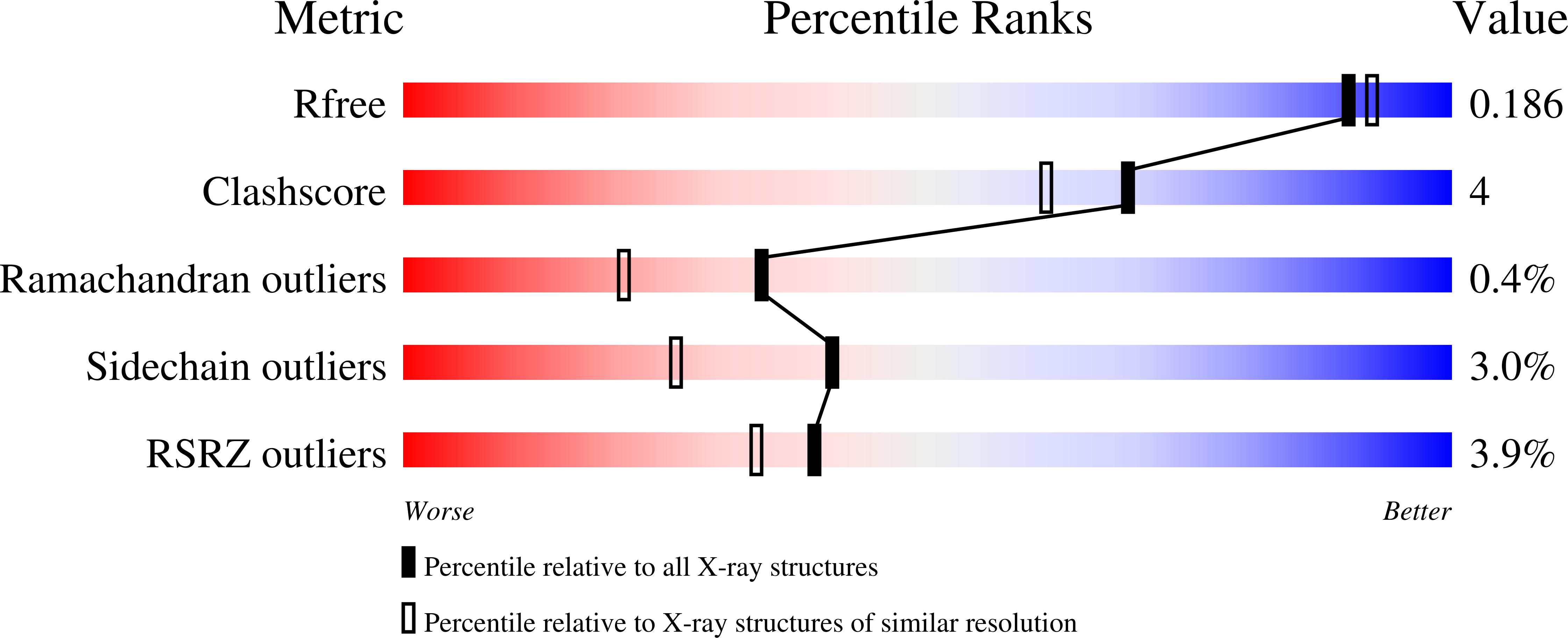
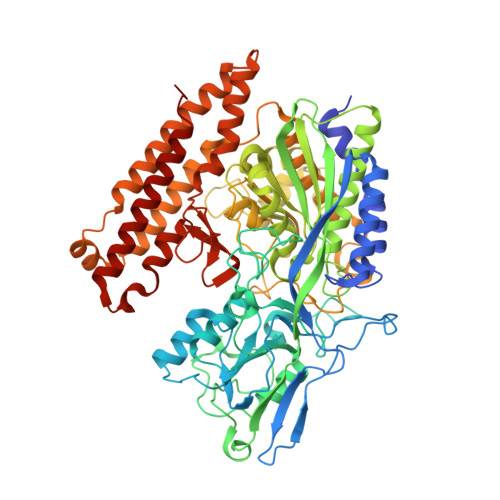
5OF0, 6H7Y, 6H7Z, 6HKJ, 6HKZ - PubMed Abstract:
The design and synthesis of prostate specific membrane antigen (PSMA) ligands derived from 2-aminoadipic acid, a building block that has not previously been used to construct PSMA ligands, are reported. The effects of both the linker length and of an N-substituent of our PSMA ligands were probed, and X-ray structures of five of these ligands bound to PSMA were obtained. Among the ligands disclosed herein, 13b showed the highest inhibitory activity for PSMA. As ligand 13b can readily be radiolabeled since its fluorine atom is adjacent to the nitrogen atom of its pyridine ring, the use of this and related compounds as theranostics can be pursued.
Organizational Affiliation:
Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago, Chicago, Illinois 60612, United States.