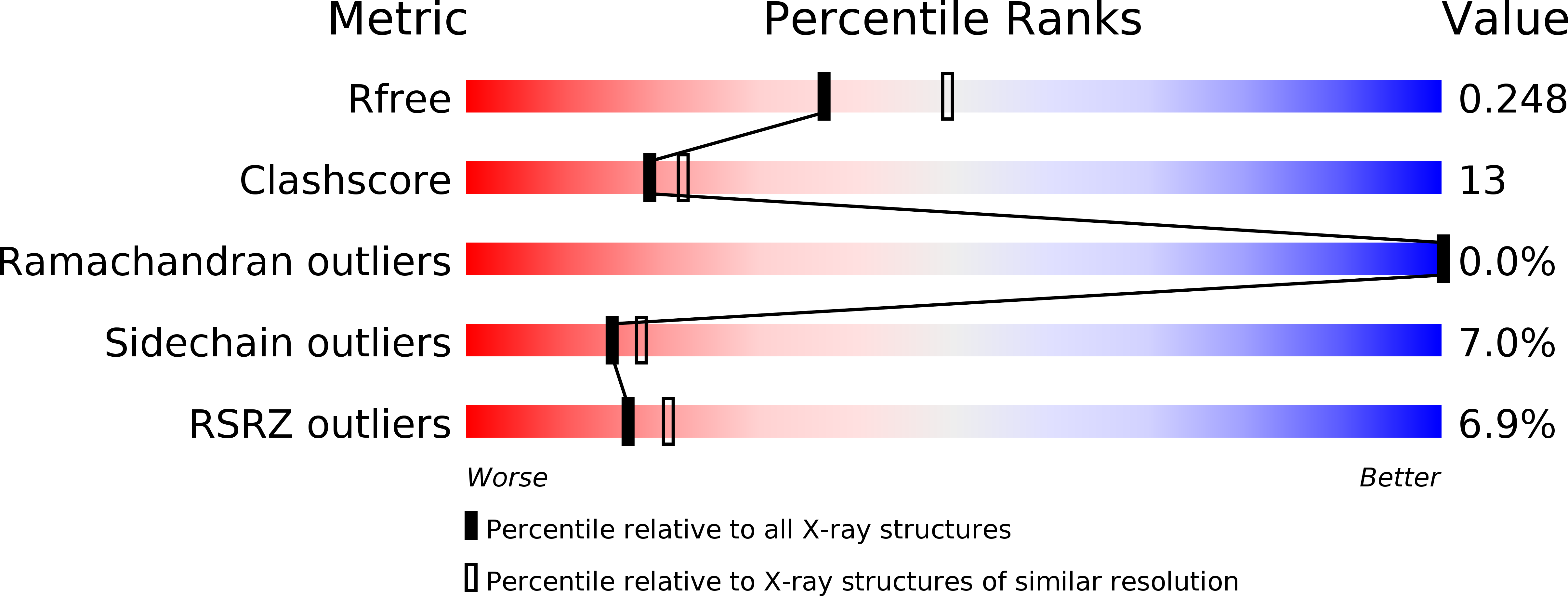
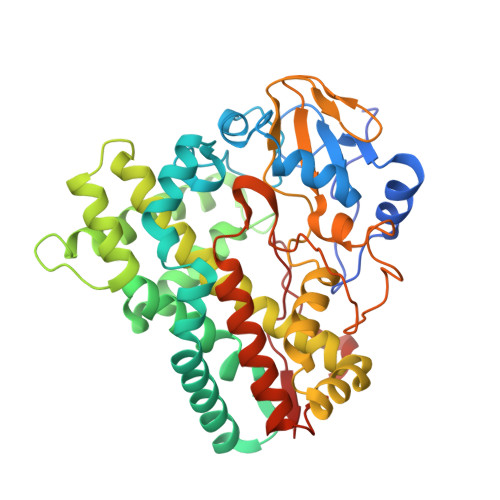
Dissecting the Cytochrome P450 OleP Substrate Specificity: Evidence for a Preferential Substrate.
Parisi, G., Freda, I., Exertier, C., Cecchetti, C., Gugole, E., Cerutti, G., D'Auria, L., Macone, A., Vallone, B., Savino, C., Montemiglio, L.C.(2020) Biomolecules 10
- PubMed: 33036250
- DOI: https://doi.org/10.3390/biom10101411
- Primary Citation of Related Structures:
6ZHZ, 6ZI2, 6ZI3, 6ZI7 - PubMed Abstract:
The cytochrome P450 OleP catalyzes the epoxidation of aliphatic carbons on both the aglycone 8.8a-deoxyoleandolide (DEO) and the monoglycosylated L-olivosyl-8.8a-deoxyoleandolide (L-O-DEO) intermediates of oleandomycin biosynthesis. We investigated the substrate versatility of the enzyme. X-ray and equilibrium binding data show that the aglycone DEO loosely fits the OleP active site, triggering the closure that prepares it for catalysis only on a minor population of enzyme. The open-to-closed state transition allows solvent molecules to accumulate in a cavity that forms upon closure, mediating protein-substrate interactions. In silico docking of the monoglycosylated L-O-DEO in the closed OleP-DEO structure shows that the L-olivosyl moiety can be hosted in the same cavity, replacing solvent molecules and directly contacting structural elements involved in the transition. X-ray structures of aglycone-bound OleP in the presence of L-rhamnose confirm the cavity as a potential site for sugar binding. All considered, we propose L-O-DEO as the optimal substrate of OleP, the L-olivosyl moiety possibly representing the molecular wedge that triggers a more efficient structural response upon substrate binding, favoring and stabilizing the enzyme closure before catalysis. OleP substrate versatility is supported by structural solvent molecules that compensate for the absence of a glycosyl unit when the aglycone is bound.
Organizational Affiliation:
Istituto Pasteur-Fondazione Cenci Bolognetti and Department of Biochemical Sciences "Alessandro Rossi Fanelli", Sapienza, University of Rome, P. le Aldo Moro, 5, 00185 Rome, Italy.