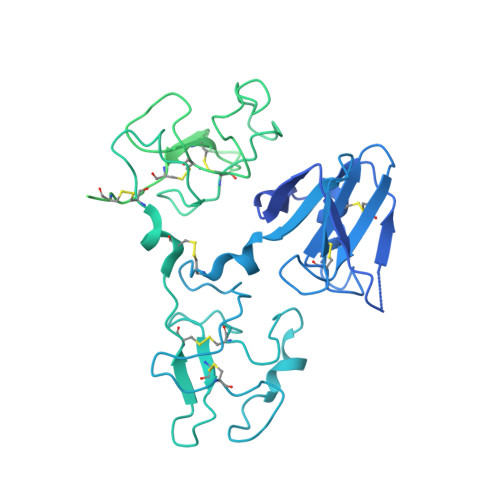
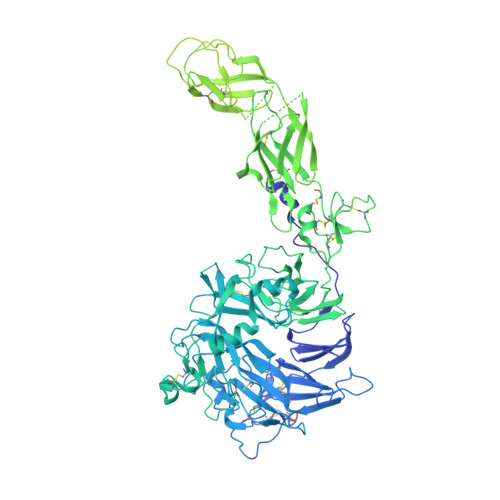
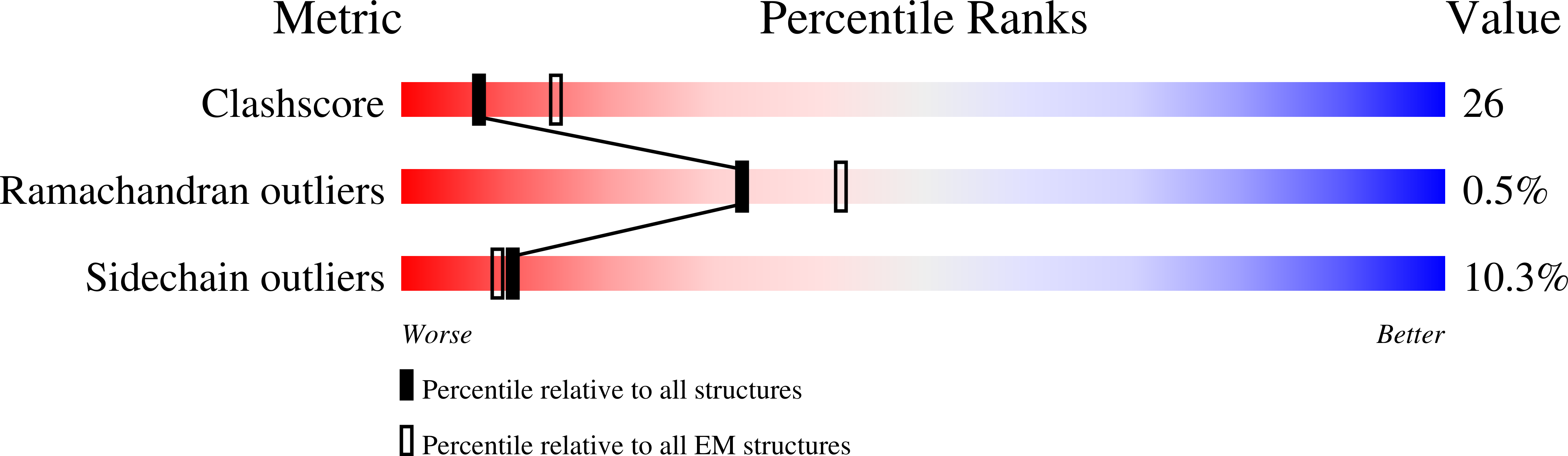Structural basis of the activation of c-MET receptor.
Uchikawa, E., Chen, Z., Xiao, G.Y., Zhang, X., Bai, X.C.(2021) Nat Commun 12: 4074-4074
- PubMed: 34210960
- DOI: https://doi.org/10.1038/s41467-021-24367-3
- Primary Citation of Related Structures:
7MO7, 7MO8, 7MO9, 7MOA, 7MOB - PubMed Abstract:
The c-MET receptor is a receptor tyrosine kinase (RTK) that plays essential roles in normal cell development and motility. Aberrant activation of c-MET can lead to both tumors growth and metastatic progression of cancer cells. C-MET can be activated by either hepatocyte growth factor (HGF), or its natural isoform NK1. Here, we report the cryo-EM structures of c-MET/HGF and c-MET/NK1 complexes in the active state. The c-MET/HGF complex structure reveals that, by utilizing two distinct interfaces, one HGF molecule is sufficient to induce a specific dimerization mode of c-MET for receptor activation. The binding of heparin as well as a second HGF to the 2:1 c-MET:HGF complex further stabilize this active conformation. Distinct to HGF, NK1 forms a stable dimer, and bridges two c-METs in a symmetrical manner for activation. Collectively, our studies provide structural insights into the activation mechanisms of c-MET, and reveal how two isoforms of the same ligand use dramatically different mechanisms to activate the receptor.
Organizational Affiliation:
Department of Biophysics, University of Texas Southwestern Medical Center, Dallas, TX, USA.