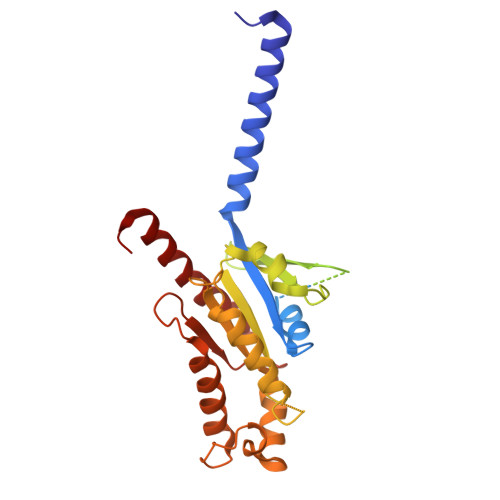
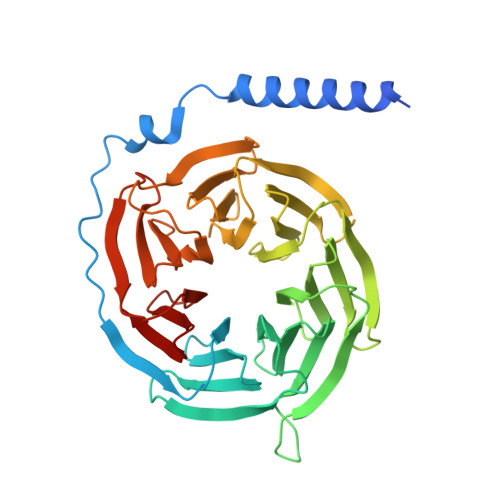
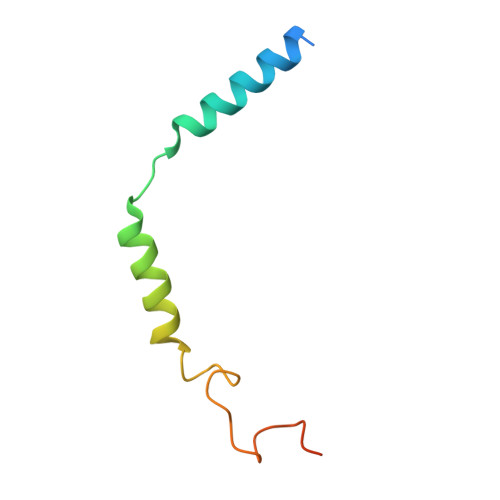
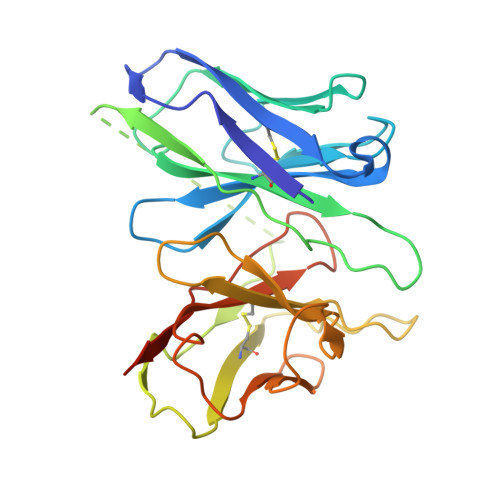
Structural insights into lipid chain-length selectivity and allosteric regulation of FFA2.
Kugawa, M., Kawakami, K., Kise, R., Suomivuori, C.M., Tsujimura, M., Kobayashi, K., Kojima, A., Inoue, W.J., Fukuda, M., Matsui, T.E., Fukunaga, A., Koyanagi, J., Kim, S., Ikeda, H., Yamashita, K., Saito, K., Ishikita, H., Dror, R.O., Inoue, A., Kato, H.E.(2025) Nat Commun 16: 2809-2809
- PubMed: 40140663
- DOI: https://doi.org/10.1038/s41467-025-57983-4
- Primary Citation of Related Structures:
8Y6W, 8Y6Y - PubMed Abstract:
The free fatty acid receptor 2 (FFA2) is a G protein-coupled receptor (GPCR) that selectively recognizes short-chain fatty acids to regulate metabolic and immune functions. As a promising therapeutic target, FFA2 has been the focus of intensive development of synthetic ligands. However, the mechanisms by which endogenous and synthetic ligands modulate FFA2 activity remain unclear. Here, we present the structures of the human FFA2-Gi complex activated by the synthetic orthosteric agonist TUG-1375 and the positive allosteric modulator/allosteric agonist 4-CMTB, along with the structure of the inactive FFA2 bound to the antagonist GLPG0974. Structural comparisons with FFA1 and mutational studies reveal how FFA2 selects specific fatty acid chain lengths. Moreover, our structures reveal that GLPG0974 functions as an allosteric antagonist by binding adjacent to the orthosteric pocket to block agonist binding, whereas 4-CMTB binds the outer surface of transmembrane helices 6 and 7 to directly activate the receptor. Supported by computational and functional studies, these insights illuminate diverse mechanisms of ligand action, paving the way for precise GPCR-targeted drug design.
- Research Center for Advanced Science and Technology, The University of Tokyo, Meguro, Tokyo, Japan.
Organizational Affiliation: