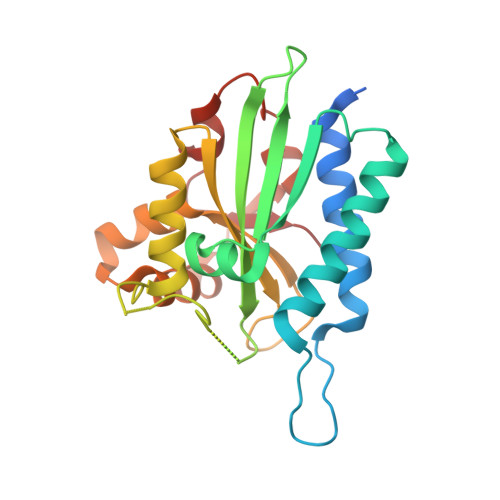
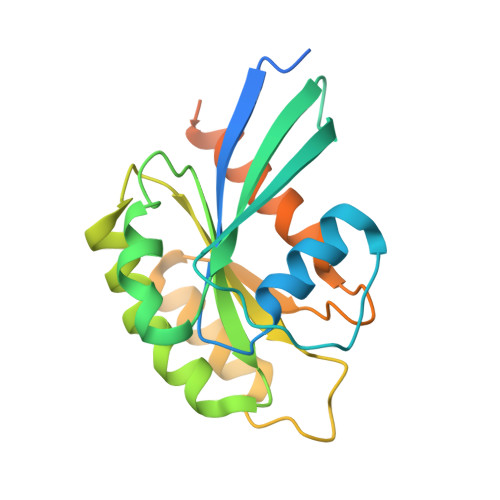
Rep15 interacts with several Rab GTPases and has a distinct fold for a Rab effector.
Rai, A., Singh, A.K., Bleimling, N., Posern, G., Vetter, I.R., Goody, R.S.(2022) Nat Commun 13: 4262-4262
- PubMed: 35871249
- DOI: https://doi.org/10.1038/s41467-022-31831-1
- Primary Citation of Related Structures:
8A4A, 8A4B, 8A4C - PubMed Abstract:
In their GTP-bound (active) form, Rab proteins interact with effector proteins that control downstream signaling. One such Rab15 effector is Rep15, which is known to have a role in receptor recycling from the endocytic recycling compartment but otherwise remains poorly characterized. Here, we report the characterization of the Rep15:Rab15 interaction and identification of Rab3 paralogs and Rab34 as Rep15 interacting partners from a yeast two-hybrid assay. Biochemical validation of the interactions is presented and crystal structures of the Rep15:Rab3B and Rep15:Rab3C complexes provide additional mechanistic insight. We find that Rep15 adopts a globular structure that is distinct from other reported Rab15, Rab3 and Rab34 effectors. Structure-based mutagenesis experiments explain the Rep15:Rab interaction specificity. Rep15 depletion in U138MG glioblastoma cells impairs cell proliferation, cell migration and receptor recycling, underscoring the need for further clarification of the role of Rep15 in cancer.
- Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, Dortmund, Germany. amrita.rai@mpi-dortmund.mpg.de.
Organizational Affiliation: