The structure of an amyloid precursor protein/talin complex indicates a mechanical basis of Alzheimer's disease.
Ellis, C., Ward, N.L., Rice, M., Ball, N.J., Walle, P., Najdek, C., Kilinc, D., Lambert, J.C., Chapuis, J., Goult, B.T.(2024) Open Biol 14: 240185-240185
- PubMed: 39591990
- DOI: https://doi.org/10.1098/rsob.240185
- Primary Citation of Related Structures:
8S4Y - PubMed Abstract:
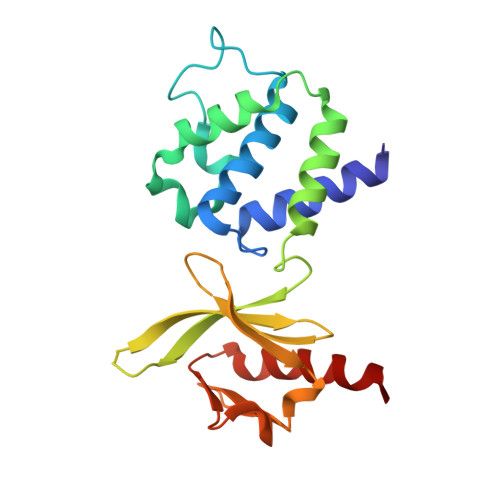
Misprocessing of amyloid precursor protein (APP) is one of the major causes of Alzheimer's disease. APP comprises a large extracellular region, a single transmembrane helix and a short cytoplasmic tail containing an NPxY motif (normally referred to as the YENPTY motif). Talins are synaptic scaffold proteins that connect the cytoskeletal machinery to the plasma membrane via binding NPxY motifs in the cytoplasmic tail of integrins. Here, we report the crystal structure of an APP/talin1 complex identifying a new way to couple the cytoskeletal machinery to synaptic sites through APP. Proximity ligation assay (PLA) confirmed the close proximity of talin1 and APP in primary neurons, and talin1 depletion had a dramatic effect on APP processing in cells. Structural modelling reveals APP might form an extracellular meshwork that mechanically couples the cytoskeletons of the pre- and post-synaptic compartments. We propose APP processing represents a mechanical signalling pathway whereby under tension, the cleavage sites in APP have varying accessibility to cleavage by secretases. This leads us to propose a new hypothesis for Alzheimer's, where misregulated APP dynamics result in loss of the mechanical integrity of the synapse, corruption and loss of mechanical binary data, and excessive generation of toxic plaque-forming Aβ42 peptide.
- School of Biosciences, University of Kent, Canterbury, Kent CT2 7NJ, UK.
Organizational Affiliation: