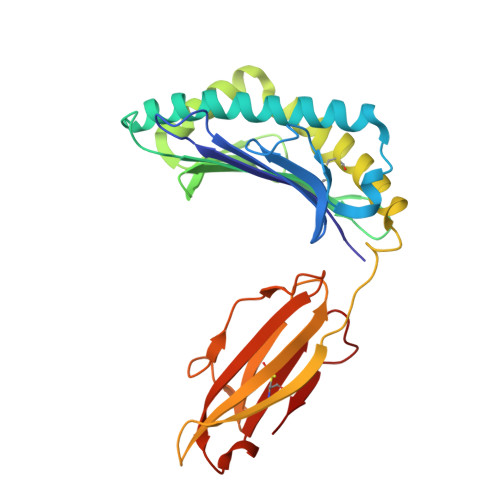
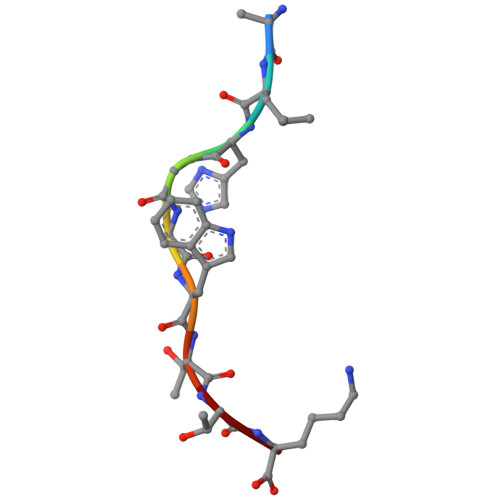
Dynamic allostery in the peptide/MHC complex enables TCR neoantigen selectivity.
Ma, J., Ayres, C.M., Brambley, C.A., Chandran, S.S., Rosales, T.J., Perera, W.W.J.G., Eldaly, B., Murray, W.T., Corcelli, S.A., Kovrigin, E.L., Klebanoff, C.A., Baker, B.M.(2025) Nat Commun 16: 849-849
- PubMed: 39833157
- DOI: https://doi.org/10.1038/s41467-025-56004-8
- Primary Citation of Related Structures:
8VCL, 9ASG - PubMed Abstract:
The inherent antigen cross-reactivity of the T cell receptor (TCR) is balanced by high specificity. Surprisingly, TCR specificity often manifests in ways not easily interpreted from static structures. Here we show that TCR discrimination between an HLA-A*03:01 (HLA-A3)-restricted public neoantigen and its wild-type (WT) counterpart emerges from distinct motions within the HLA-A3 peptide binding groove that vary with the identity of the peptide's first primary anchor. These motions create a dynamic gate that, in the presence of the WT peptide, impedes a large conformational change required for TCR binding. The neoantigen is insusceptible to this limiting dynamic, and, with the gate open, upon TCR binding the central tryptophan can transit underneath the peptide backbone to the opposing side of the HLA-A3 peptide binding groove. Our findings thus reveal a novel mechanism driving TCR specificity for a cancer neoantigen that is rooted in the dynamic and allosteric nature of peptide/MHC-I binding grooves, with implications for resolving long-standing and often confounding questions about T cell specificity.
- Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN, USA.
Organizational Affiliation: