NP-LCK2-1TCR-MHC complex
Zhang, J.B., Chaurasia, P., Littler, D.R., La Gruta, N., Rossjohn, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| H-2 class II histocompatibility antigen, A-B alpha chain | A [auth C] | 188 | Mus musculus | Mutation(s): 0 Gene Names: H2-Aa | 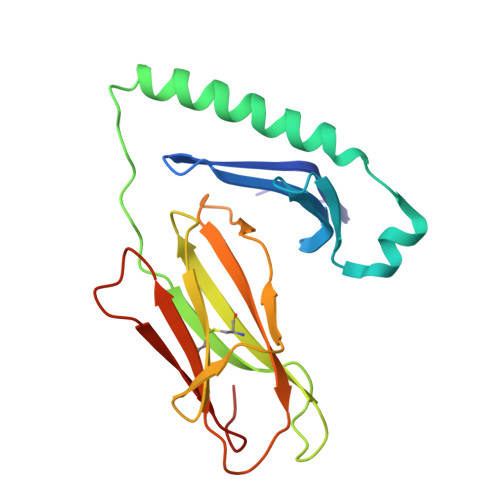 |
UniProt | |||||
Find proteins for P14434 (Mus musculus) Explore P14434 Go to UniProtKB: P14434 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14434 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | |||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nucleoprotein,H-2 class II histocompatibility antigen, A beta chain | B [auth D] | 228 | Influenza A virus H3N2, Mus musculus This entity is chimeric | Mutation(s): 0 Gene Names: NP, H2-Ab1, H2-iabeta | 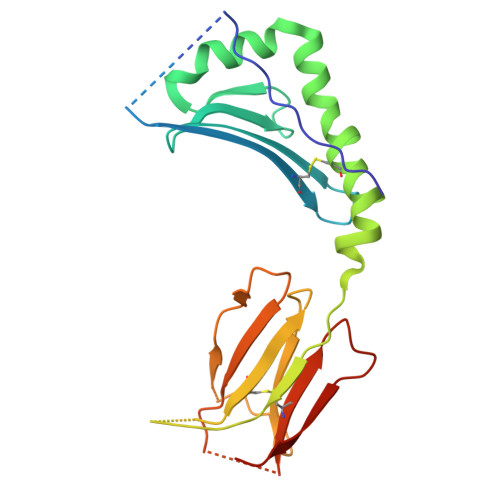 |
UniProt | |||||
Find proteins for O92607 (Influenza A virus H3N2) Explore O92607 Go to UniProtKB: O92607 | |||||
Find proteins for P14483 (Mus musculus) Explore P14483 Go to UniProtKB: P14483 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P14483O92607 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | |||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| NPLCK1-2_TCR TRAV6-5 alpha chain | C [auth A] | 210 | Mus musculus | Mutation(s): 0 | 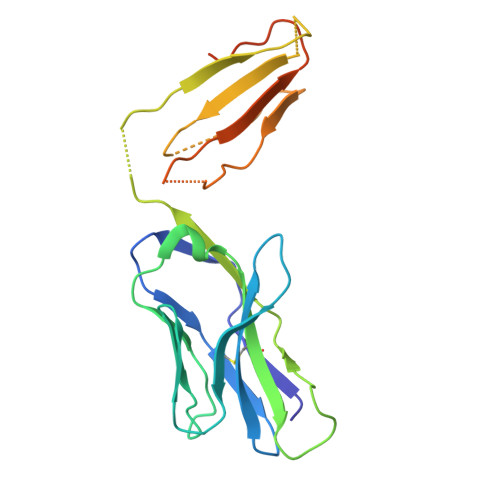 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| NPLCK1-2 TCR TRBV1 Beta chain | D [auth B] | 242 | Mus musculus | Mutation(s): 0 | 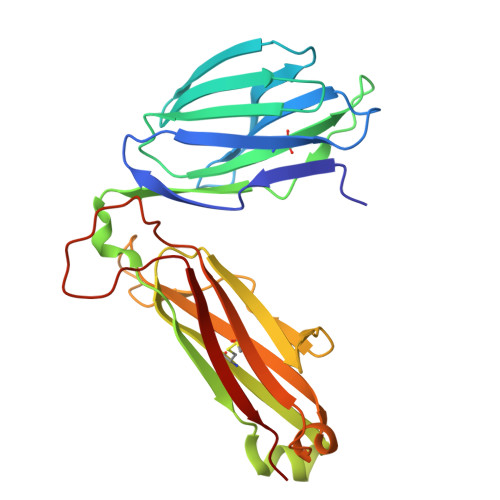 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | G [auth C], I [auth D] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| EDO Query on EDO | E [auth C] F [auth C] H [auth C] J [auth D] K [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 196.344 | α = 90 |
| b = 196.344 | β = 90 |
| c = 77.104 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Health and Medical Research Council (NHMRC, Australia) | Australia | -- |