Cryo-EM structure of Human Nucleosome collected by EPU on Glacios at 3.3 Angstrom resolution
Jia, L., Ruben, E.A., Olsen, S.K., Wasmuth, E.V.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H3.2 | 97 | Homo sapiens | Mutation(s): 0 Gene Names: H3C15, HIST2H3A, H3C14, H3F2, H3FM, HIST2H3C, H3C13, HIST2H3D | 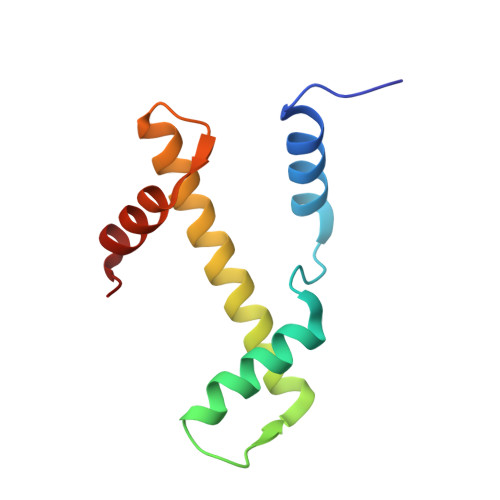 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q71DI3 (Homo sapiens) Explore Q71DI3 Go to UniProtKB: Q71DI3 | |||||
PHAROS: Q71DI3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q71DI3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H4 | 83 | Homo sapiens | Mutation(s): 0 |  | |
UniProt | |||||
Find proteins for A0A9J8D176 (Cyprinus carpio carpio) Explore A0A9J8D176 Go to UniProtKB: A0A9J8D176 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A9J8D176 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H2A type 1 | 103 | Homo sapiens | Mutation(s): 0 Gene Names: | 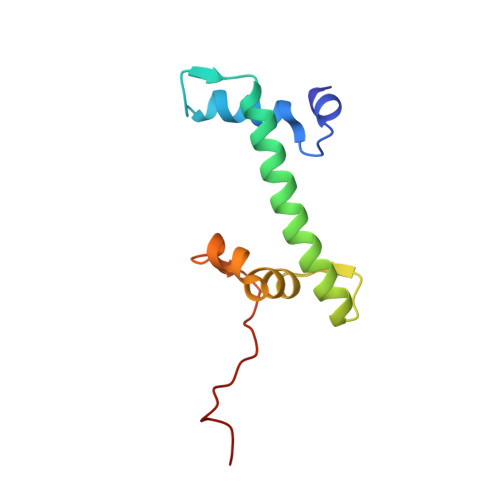 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0C0S8 (Homo sapiens) Explore P0C0S8 Go to UniProtKB: P0C0S8 | |||||
PHAROS: P0C0S8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0C0S8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H2B type 1-J | 95 | Homo sapiens | Mutation(s): 0 Gene Names: H2BC11, H2BFR, HIST1H2BJ | 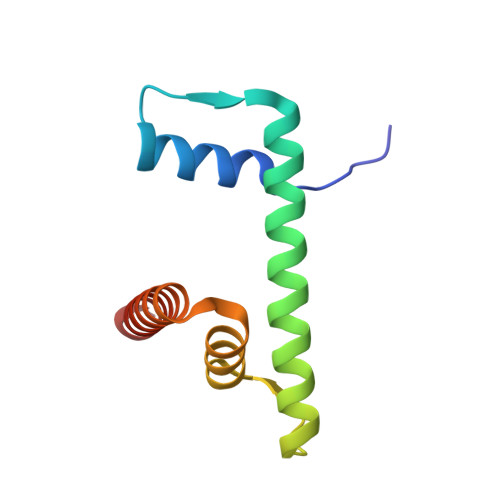 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P06899 (Homo sapiens) Explore P06899 Go to UniProtKB: P06899 | |||||
PHAROS: P06899 GTEx: ENSG00000124635 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06899 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H3.2 | 98 | Homo sapiens | Mutation(s): 0 Gene Names: H3C15, HIST2H3A, H3C14, H3F2, H3FM, HIST2H3C, H3C13, HIST2H3D | 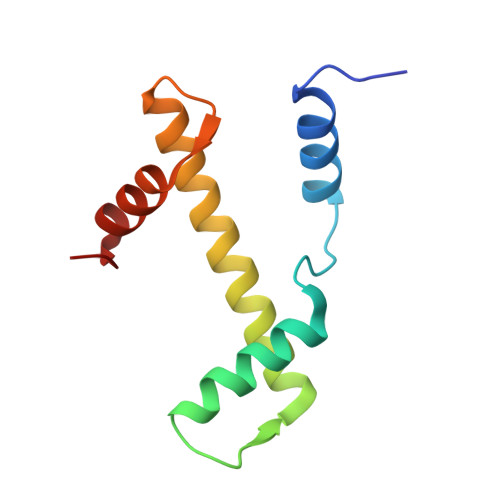 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q71DI3 (Homo sapiens) Explore Q71DI3 Go to UniProtKB: Q71DI3 | |||||
PHAROS: Q71DI3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q71DI3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H2A type 1-B/E | 105 | Homo sapiens | Mutation(s): 0 Gene Names: H2AC4, H2AFM, HIST1H2AB, H2AC8, H2AFA, HIST1H2AE |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P04908 (Homo sapiens) Explore P04908 Go to UniProtKB: P04908 | |||||
PHAROS: P04908 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P04908 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 7 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (145-MER) | 145 | Homo sapiens |  | ||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 8 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (145-MER) | 145 | Homo sapiens |  | ||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | -- |