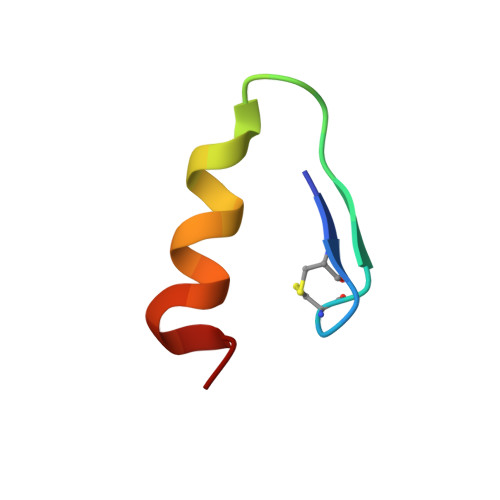
Solution structure of the Z0 domain from transcription repressor BCL11A sheds light on the sequence properties of protein-binding zinc fingers.
Harris, R.E., Whitehead 3rd, R.D., Alexandrescu, A.T.(2025) Protein Sci 34: e70097-e70097
- PubMed: 40099876
- DOI: https://doi.org/10.1002/pro.70097
- Primary Citation of Related Structures:
9BV0 - PubMed Abstract:
The transcription repressor BCL11A governs the switch from fetal to adult hemoglobin during development. By targeting BCL11A, fetal hemoglobin expression can be de-repressed to substitute for defective adult hemoglobin in inherited diseases including beta-thalassemia and sickle-cell anemia. BCL11A has six CCHH-type zinc fingers, of which domains 4-6 are necessary and sufficient for dsDNA binding. Here, we focus on a putative ZNF at the N-terminus of BCL11A (residues 46-72), Z0, thought to modulate oligomerization of the transcription repressor. Using NMR and CD spectroscopy at low concentrations that favor the monomer, Z0 is shown to be a thermostable CCHC zinc finger with a pM dissociation constant for zinc. The NMR structure of Z0 has a prototypical beta-beta-alpha fold, with a hydrophobic knob comprising about half the structure. The unusual proportion of hydrophobic residues in Z0 led us to investigate if this is a more general feature of zinc fingers that do not bind dsDNA. We used the ZF and WebLogo servers to examine sequences of zinc fingers with demonstrated DNA-binding function, non-DNA-binders, and the CCHC-type family of protein-binders. DNA-binders are distinguished by contiguous stretches of high-scoring zinc fingers. Non-DNA-binders show a depletion of polar residues at the positions expected to contact nucleotides and increased sequence divergence, making these domains more likely to be annotated as atypical, degenerate, or to be missed as zinc fingers. We anticipate these sequence patterns will help distinguish DNA-binders from non-binders, an open problem in the functional understanding of zinc-finger motifs.
- Department of Molecular and Cellular Biology, University of Connecticut, Storrs, Connecticut, USA.
Organizational Affiliation: