Structural basis for transcription activation through cooperative recruitment of MntR.
Shi, H., Fu, Y., Kodyte, V., Andreas, A., Sachla, A.J., Miller, K., Shrestha, R., Helmann, J.D., Glasfeld, A., Ahuja, S.(2025) Nat Commun 16: 2204-2204
- PubMed: 40044701
- DOI: https://doi.org/10.1038/s41467-025-57412-6
- Primary Citation of Related Structures:
9C4C, 9C4D - PubMed Abstract:
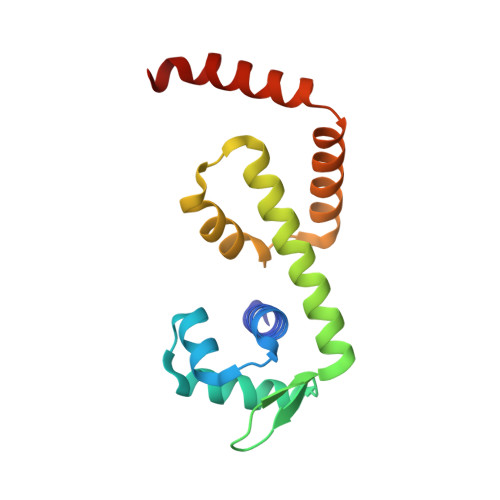
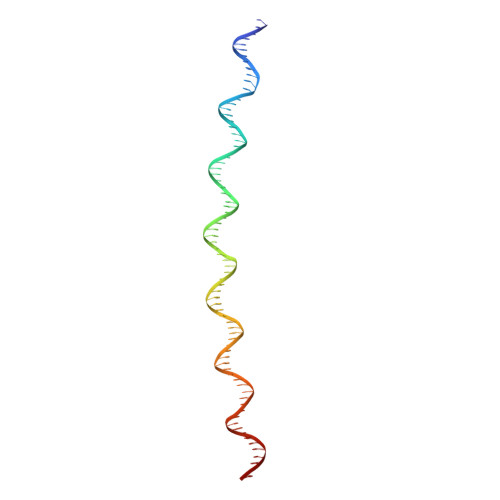
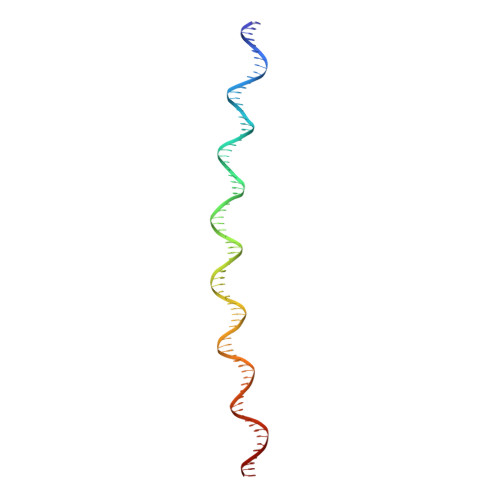
Bacillus subtilis MntR is a dual regulatory protein that responds to heightened Mn 2+ availability in the cell by both repressing the expression of uptake transporters and activating the expression of efflux proteins. Recent work indicates that, in its role as an activator, MntR binds several sites upstream of the genes encoding Mn 2+ exporters, leading to a cooperative response to manganese. Here, we use cryo-EM to explore the molecular basis of gene activation by MntR and report a structure of four MntR dimers bound to four 18-base pair sites across an 84-base pair regulatory region of the mneP promoter. Our structures, along with solution studies including mass photometry and in vivo transcription assays, reveal that MntR dimers employ polar and non-polar contacts to bind cooperatively to an array of low-affinity DNA-binding sites. These results reveal the molecular basis for cooperativity in the activation of manganese efflux.
- Department of Chemistry, Reed College, Portland, Oregon, USA.
Organizational Affiliation: