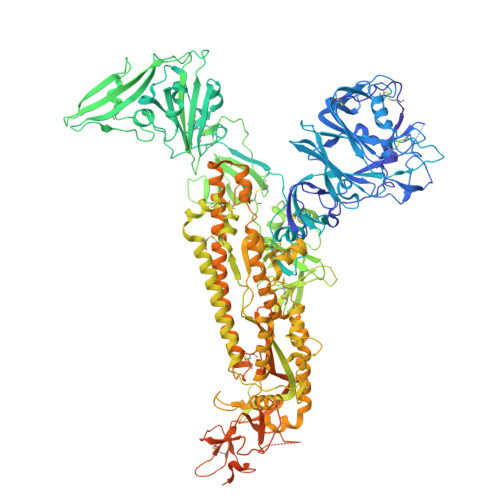
ACE2 from Pipistrellus abramus bats is a receptor for HKU5 coronaviruses.
Catanzaro, N.J., Wu, Z., Fan, C., Jefferson, V., Abdelgadir, A., Schafer, A., Yount, B.L., Bjorkman, P.J., Baric, R., Letko, M.(2025) Nat Commun 16: 4932-4932
- PubMed: 40436893
- DOI: https://doi.org/10.1038/s41467-025-60286-3
- Primary Citation of Related Structures:
9D4T - PubMed Abstract:
The merbecovirus subgenus of coronaviruses includes Middle East Respiratory Syndrome Coronavirus (MERS-CoV), a zoonotic pathogen transmitted from dromedary camels to humans that causes severe respiratory disease. Viral discovery efforts uncover hundreds of merbecoviruses in different species across multiple continents, but few are studied under laboratory conditions, leaving basic questions regarding their human threat potential unresolved. Viral entry into host cells is a critical step for transmission between hosts. Here, we develop and apply a scalable approach to assesses novel merbecovirus cell entry across the entire merbecovirus subgenus. Merbecoviruses are sorted into clades based on the receptor-binding domain of the spike glycoprotein. Receptor tropism is clade-specific, with the clade including MERS-CoV using DPP4 and multiple clades using ACE2, including HKU5 bat coronaviruses. Mutational analysis identifies possible structural limitations to HKU5 adaptability and a cryo-EM structure of the HKU5-20s spike trimer reveals only 'down' RBDs.
- Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Organizational Affiliation: