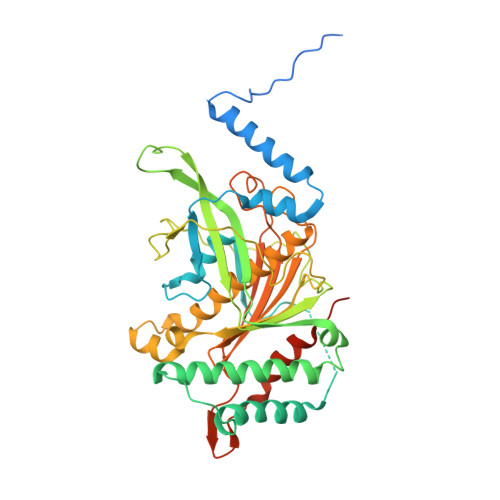
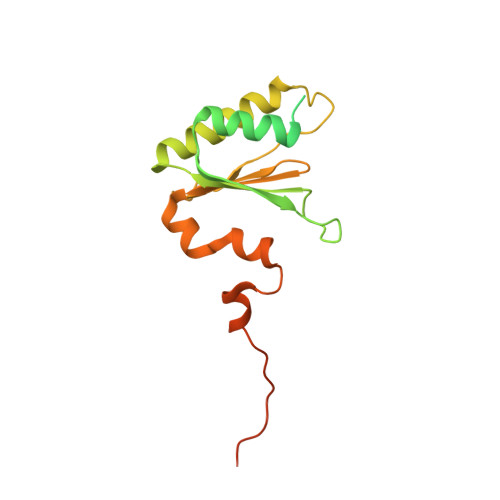
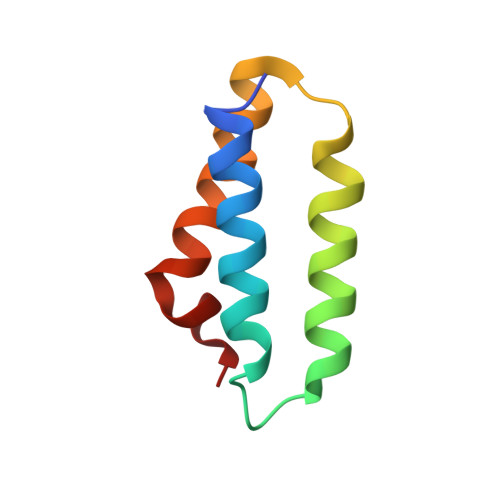
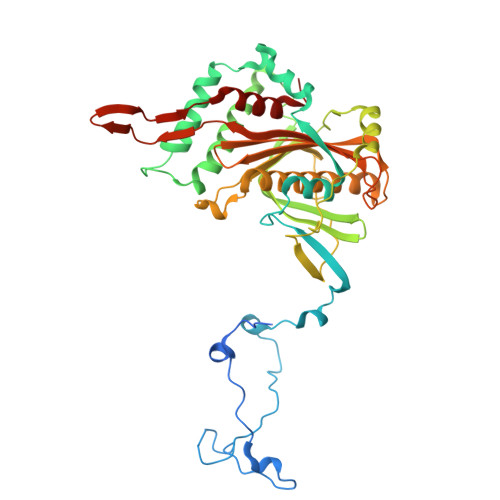
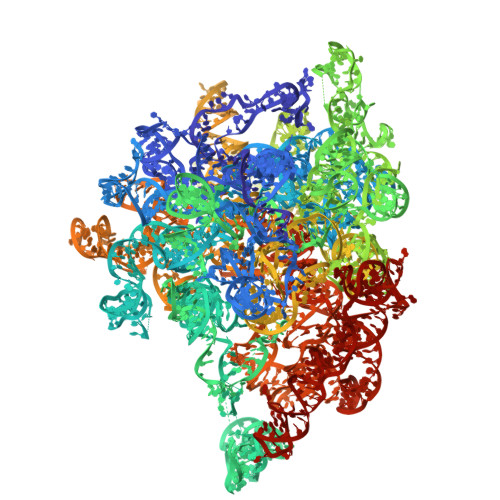
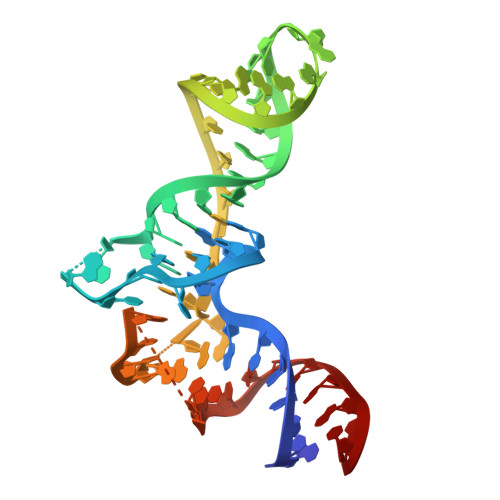
The mitochondrial methylation potential gates mitoribosome assembly.
Glasgow, R.I.C., Singh, V., Pena-Perez, L., Wilhalm, A., Moedas, M.F., Moore, D., Rosenberger, F.A., Li, X., Atanassov, I., Saba, M., Cipullo, M., Rorbach, J., Wedell, A., Freyer, C., Amunts, A., Wredenberg, A.(2025) Nat Commun 16: 5388-5388
- PubMed: 40562754
- DOI: https://doi.org/10.1038/s41467-025-60977-x
- Primary Citation of Related Structures:
9HCC, 9HCD, 9HCE, 9HCF, 9HCG, 9HCH - PubMed Abstract:
S-adenosylmethionine (SAM) is the principal methyl donor in cells and is essential for mitochondrial gene expression, influencing RNA modifications, translation, and ribosome biogenesis. Using direct long-read RNA sequencing in mouse tissues and embryonic fibroblasts, we show that processing of the mitochondrial ribosomal gene cluster fails in the absence of mitochondrial SAM, leading to an accumulation of unprocessed precursors. Proteomic analysis of ribosome fractions revealed these precursors associated with processing and assembly factors, indicating stalled biogenesis. Structural analysis by cryo-electron microscopy demonstrated that SAM-dependent methylation is required for peptidyl transferase centre formation during mitoribosome assembly. Our findings identify a critical role for SAM in coordinating mitoribosomal RNA processing and large subunit maturation, linking cellular methylation potential to mitochondrial translation capacity.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden.