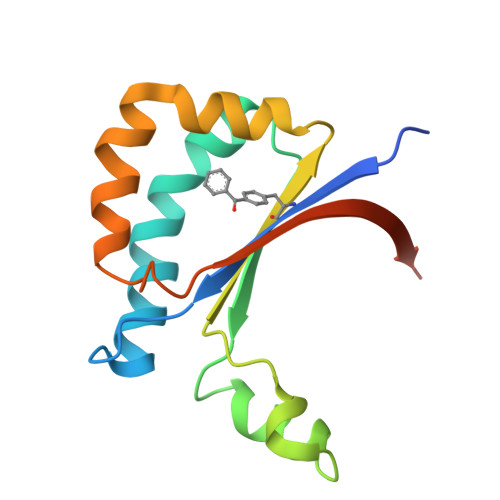
Light-Driven Deracemization by a Designed Photoenzyme.
Li, M., Zhang, Y., Fu, K., Deng, Z., Yuan, Z., Luo, Z., Rao, Y.(2025) J Am Chem Soc 147: 13190-13199
- PubMed: 40219972
- DOI: https://doi.org/10.1021/jacs.4c16521
- Primary Citation of Related Structures:
9IKU, 9ILO, 9IM9, 9IPR - PubMed Abstract:
The creation of enzymes with abiological abilities offers exciting opportunities to access new-to-nature biocatalysis beyond that found in nature. Here, we repurpose a novel protein scaffold, CTB10, as an artificial photoenzyme through genetic code expansion. It enables catalytic deracemization of cyclopropane, a process that remains inaccessible to traditional biocatalysis due to its thermodynamically unfavorable nature. Following structural optimization through directed evolution, a broad substrate scope with high enantioselectivities is achieved. Furthermore, the crystal structure of the CTB10-based photoenzyme-substrate complex well demonstrates how the catalytic chiral cavity is sculpted to promote efficient and selective light-enabled deracemization. Therefore, this study unlocks the potential for achieving challenging deracemization through biocatalysis.
- Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, P. R. China.
Organizational Affiliation: