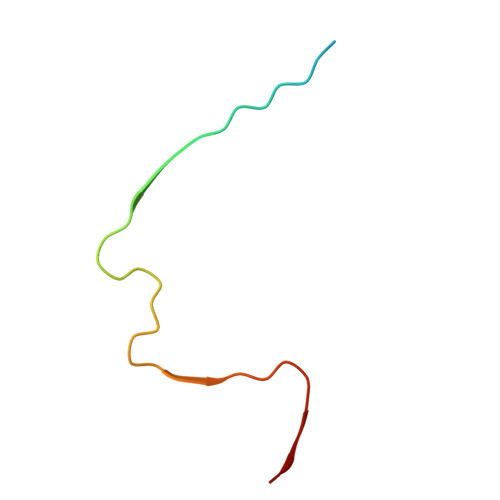
An O-glycopeptide participates in the formation of distinct A beta 42 fibril structures and attenuates A beta 42 neurotoxicity.
Wei, Q., Liu, D., Xia, W., Wang, F., Huang, L., Zhang, J., Wang, X., Xu, Z., He, C., Li, W., Shi, X., Wang, C., Liu, Y., Liu, C., Dong, S.(2025) Nat Commun 16: 5815-5815
- PubMed: 40593714
- DOI: https://doi.org/10.1038/s41467-025-60978-w
- Primary Citation of Related Structures:
9K0D, 9K0E, 9K0F - PubMed Abstract:
The self-assembly of biomolecules through noncovalent interactions is critical in both physiological and pathological processes, as exemplified by the assembly of amyloid β peptide (Aβ) into oligomers or fibrils in Alzheimer's disease (AD). Developing molecules that can modulate this assembly process holds significant mechanistic and therapeutic potential. In this study, we identified glycopeptides as a class of protein aggregation modulators, showing that β-N-acetylgalactosamine (β-GalNAc)-modified Aβ 9-21 promotes Aβ 42 fibrillation while reducing its toxic oligomers. Using biochemical assays, cryo-EM, and molecular dynamics simulations, we demonstrated that β-GalNAc-modified Aβ 9-21 coassembles with Aβ 42 , forming unique fibril structures stabilized by both hydrophobic interactions and an organized hydrogen bond network facilitated by the glycopeptide. Importantly, β-GalNAc-modified Aβ 9-21 can alleviate the neurotoxicity of Aβ 42 in neuronal cells and an AD male mouse model. These findings underscore the potential of glycopeptides in regulating amyloid aggregation and provide structural insights for designing molecules targeting amyloid-related pathologies.
- State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing, China.
Organizational Affiliation: