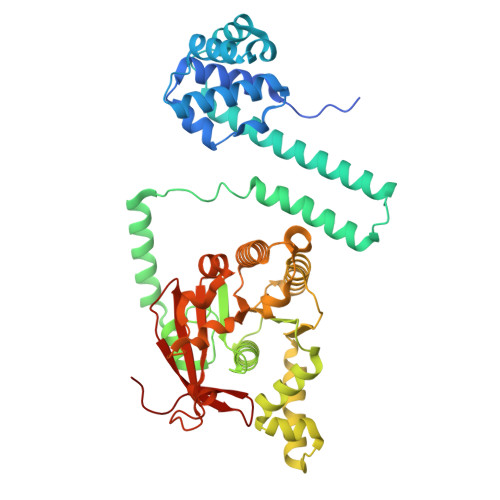
Distinct Quaternary States, Intermediates, and Autoinhibition During Loading of the DnaB-Replicative Helicase by the Phage lambda P Helicase Loader.
Shatarupa, A., Brown, D., Olinares, P.D.B., Chase, J., Isiorho, E., Chait, B.T., Jeruzalmi, D.(2025) bioRxiv
- PubMed: 40501539
- DOI: https://doi.org/10.1101/2022.12.30.522210
- Primary Citation of Related Structures:
8V9S, 9OA1, 9OA2 - PubMed Abstract:
Replicative helicases require loader proteins for assembly at the origins of DNA replication. Multiple copies of the bacteriophage λP (P) loader bind to and load the E. coli DnaB (B) replicative helicase on replication-origin-derived single-stranded DNA. We find that the E. coli DnaB•λP complex exists in two forms: B 6 P 5 and B 6 P 6 . In the 2.66 Å cryo-EM model of B 6 P 5 , five copies of the λP loader assemble into a crown-like shape that tightly grips DnaB. In this complex, closed planar DnaB is reconfigured into an open spiral with a sufficiently sized breach to permit ssDNA to enter an internal chamber. The transition to the open spiral involves λP-mediated changes to the Docking Helix (DH)-Linker Helix (LH) interface. The loader directly stabilizes the open spiral. Unexpectedly, one λP chain in B 6 P 5 is bound across the breach, precluding entry of replication-origin-derived ssDNA into DnaB's central chamber. We suggest that the B 6 P 6 complex is an early intermediate in the helicase activation pathway wherein neither the DnaB helicase nor the λP loader has attained its final form. DnaB in this complex adopts a partially open planar configuration, termed ajar planar. The partially ordered λP loader assembly features a much looser interaction with DnaB. The ssDNA and ATP sites in both complexes are in a configuration ill-suited for binding or hydrolysis. Our work specifies the conformational changes required for the intermediate B 6 P 6 to transition to B 6 P 5 on the pathway to recruitment by the initiator protein complex to the replication origin.