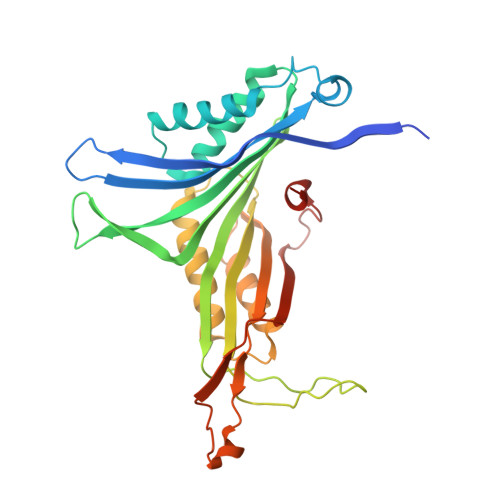
Structures of Arthrobacter globiformis urate oxidase-ligand complexes.
Juan, E.C., Hoque, M.M., Shimizu, S., Hossain, M.T., Yamamoto, T., Imamura, S., Suzuki, K., Tsunoda, M., Amano, H., Sekiguchi, T., Takenaka, A.(2008) Acta Crystallogr D Biol Crystallogr D64: 815-822
- PubMed: 18645230
- DOI: https://doi.org/10.1107/S0907444908013590
- Primary Citation of Related Structures:
2YZB, 2YZC, 2YZD, 2YZE - PubMed Abstract:
The enzyme urate oxidase catalyzes the conversion of uric acid to 5-hydroxyisourate, one of the steps in the ureide pathway. Arthrobacter globiformis urate oxidase (AgUOX) was crystallized and structures of crystals soaked in the substrate uric acid, the inhibitor 8-azaxanthin and allantoin have been determined at 1.9-2.2 A resolution. The biological unit is a homotetramer and two homotetramers comprise the asymmetric crystallographic unit. Each subunit contains two T-fold domains of betabetaalphaalphabetabeta topology, which are usually found in purine- and pterin-binding enzymes. The uric acid substrate is bound tightly to the enzyme by interactions with Arg180, Leu222 and Gln223 from one subunit and with Thr67 and Asp68 of the neighbouring subunit in the tetramer. In the other crystal structures, lithium borate, 8-azaxanthin and allantoate are bound to the enzyme in a similar manner as uric acid. Based on these AgUOX structures, the enzymatic reaction mechanism of UOX has been proposed.
- Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, Kanagawa 226-8501, Japan.
Organizational Affiliation: