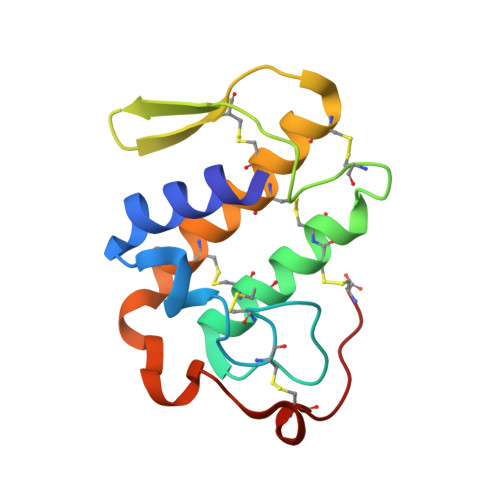
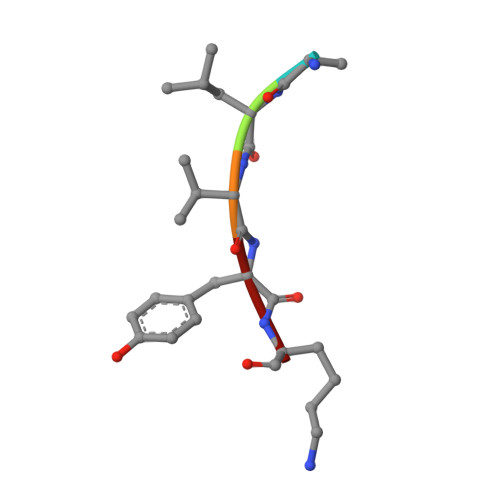
Design of specific peptide inhibitors of phospholipase A2: Crystal structure of the complex formed between a group II phospholipase A2 and a designed pentapeptide Ala - Leu - Val - Tyr - Lys at 2.3 A resolution
Singh, N., Sharma, S., Somvanshi, R.K., Dey, S., Singh, T.P.To be published.