Discovery of Novel and Ligand-Efficient Inhibitors of Plasmodium Falciparum and Plasmodium Vivax N-Myristoyltransferase.
Rackham, M.D., Brannigan, J.A., Moss, D.K., Yu, Z., Wilkinson, A.J., Holder, A.A., Tate, E.W., Leatherbarrow, R.J.(2013) J Med Chem 56: 371
- PubMed: 23170970
- DOI: https://doi.org/10.1021/jm301474t
- Primary Citation of Related Structures:
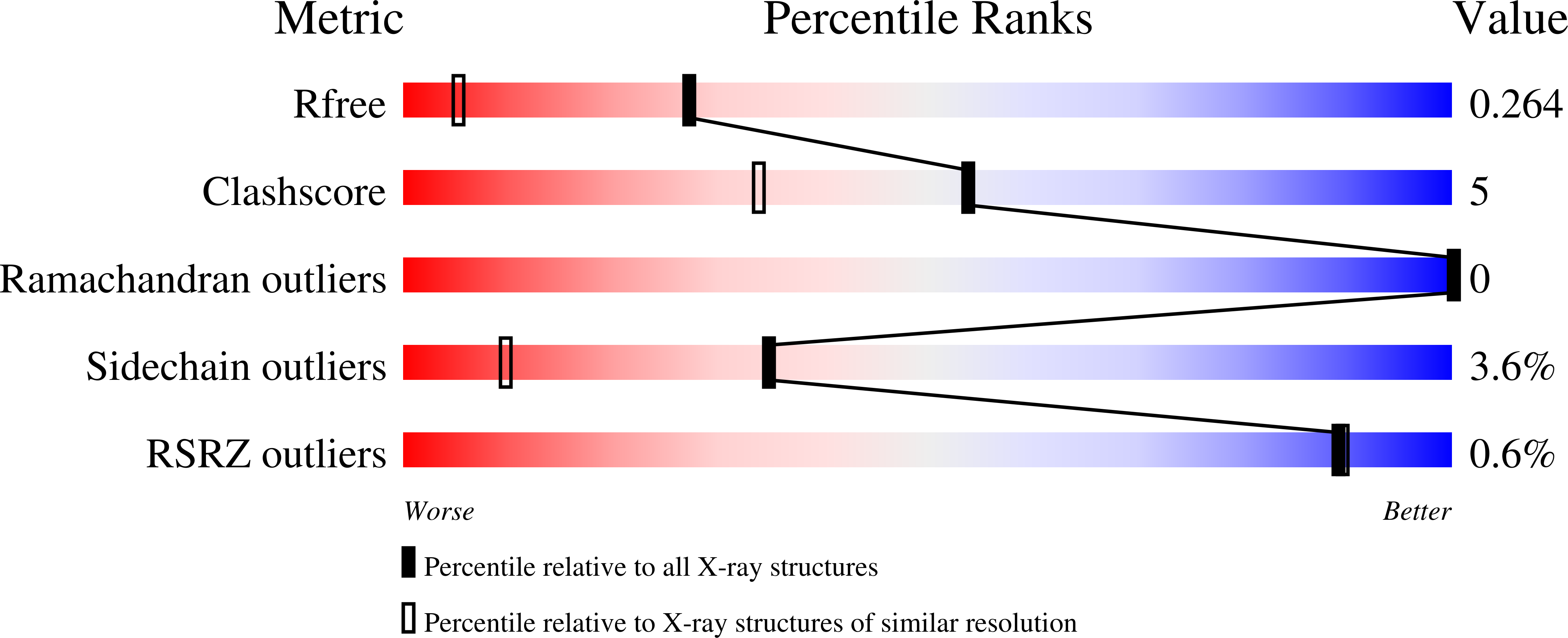
4BBH - PubMed Abstract:
N-Myristoyltransferase (NMT) is an attractive antiprotozoan drug target. A lead-hopping approach was utilized in the design and synthesis of novel benzo[b]thiophene-containing inhibitors of Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) NMT. These inhibitors are selective against Homo sapiens NMT1 (HsNMT), have excellent ligand efficiency (LE), and display antiparasitic activity in vitro. The binding mode of this series was determined by crystallography and shows a novel binding mode for the benzothiophene ring.
Organizational Affiliation:
Department of Chemistry, Imperial College London, South Kensington Campus, London, SW7 2AZ, U.K.