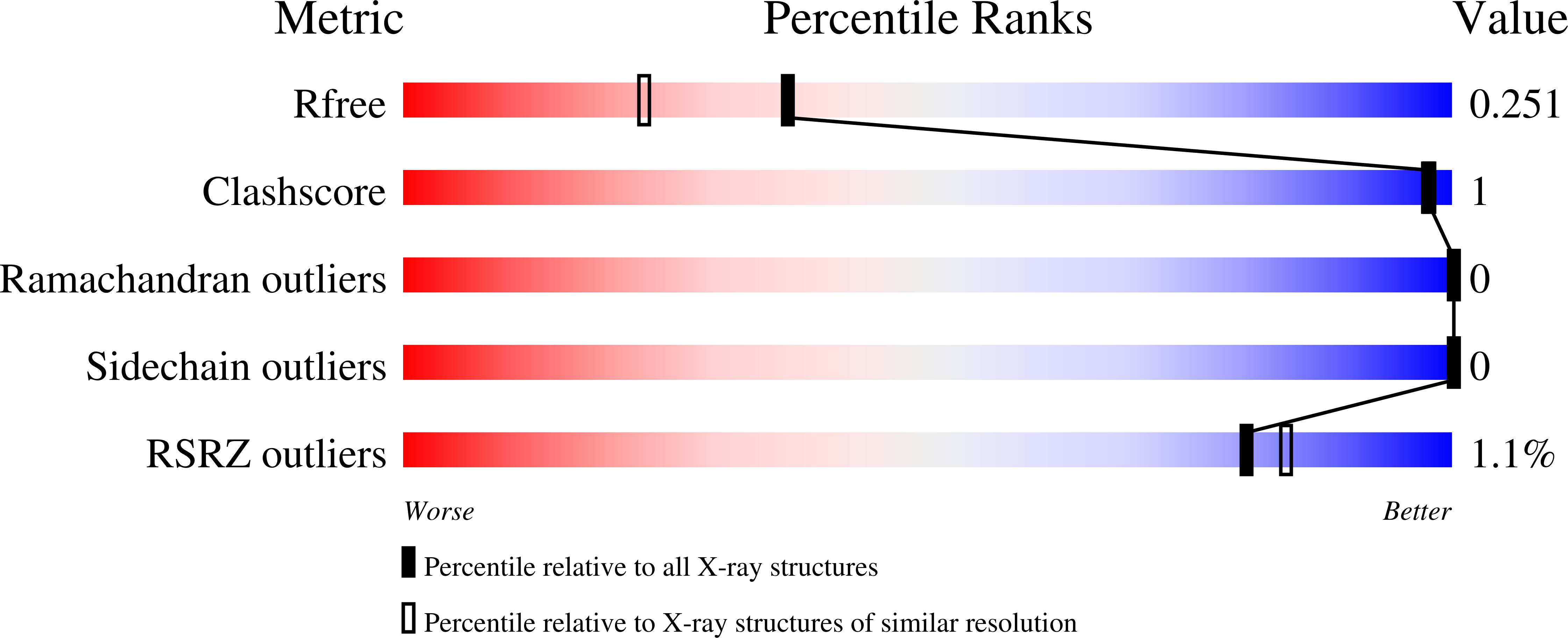
Boiling down the cysteine-stabilized LTP fold - loss of structural and immunological integrity of allergenic Art v 3 and Pru p 3 as a consequence of irreversible lanthionine formation.
Wildner, S., Griessner, I., Stemeseder, T., Regl, C., Soh, W.T., Stock, L.G., Volker, T., Alessandri, C., Mari, A., Huber, C.G., Stutz, H., Brandstetter, H., Gadermaier, G.(2019) Mol Immunol 116: 140-150
- PubMed: 31654938
- DOI: https://doi.org/10.1016/j.molimm.2019.10.012
- Primary Citation of Related Structures:
6FRR - PubMed Abstract:
Non-specific lipid transfer proteins (LTPs) are important allergens in fruits, pollen, vegetables, nuts and latex. Due to their compact structure, LTPs are highly resistant to heat treatment. Here, Art v 3 from mugwort pollen and Pru p 3 from peach were used as model allergens to in-depth investigate structural and immunological properties upon thermal treatment at different buffer conditions. Recombinant Art v 3 and Pru p 3 were purified from E. coli and incubated at 95 °C up to 120 min using sodium phosphate buffer pH 3.4 or 7.3. Physicochemical properties of allergens were analyzed in circular dichroism spectroscopy, Fourier transform infrared spectroscopy, dynamic light scattering, size exclusion chromatography, and mass spectrometry. The crystal structure of Art v 3.0201 was determined to 1.9 Å resolution. IgG and IgE binding was investigated in ELISA using murine and LTP allergic patients' sera. Highly pure and homogenous recombinant allergens were obtained from bacterial production. The crystal structure of Art v 3.0201 revealed an antiparallel four helix bundle with a C-terminal extension mediating an asymmetric, transient dimer interface and differently sized cavities. Both allergens showed high thermal stability at acidic conditions. In contrast, extensive heat treatment in neutral buffer induced irreversible structural changes due to lanthionine-based cysteine rearrangement. This fostered loss of the typical α-helical structure, increased molecular size and abrogation of IgG and IgE binding epitopes. Pru p 3 lost its structural integrity at shorter heat stress duration than Art v 3, which did however only partially affect the molecule's IgE binding epitopes. During thermal treatment, susceptibility to structural changes of the LTP-fold is highly dependent on the surrounding environment but also on intrinsic features of individual LTPs. This is a crucial fact to consider when processing LTP-containing food or food products as this will directly influence their allergenic potential.
Organizational Affiliation:
Department of Biosciences, University of Salzburg, Salzburg, Austria; Christian Doppler Laboratory for Innovative Tools for the Characterization of Biosimilars, University of Salzburg, Salzburg, Austria.