A novel cryoprotection scheme for enhancing the diffraction of crystals of recombinant cytochrome ba3 oxidase from Thermus thermophilus.
Hunsicker-Wang, L.M., Pacoma, R.L., Chen, Y., Fee, J.A., Stout, C.D.(2005) Acta Crystallogr D Biol Crystallogr 61: 340-343
- PubMed: 15735345
- DOI: https://doi.org/10.1107/S0907444904033906
- Primary Citation of Related Structures:
1XME - PubMed Abstract:
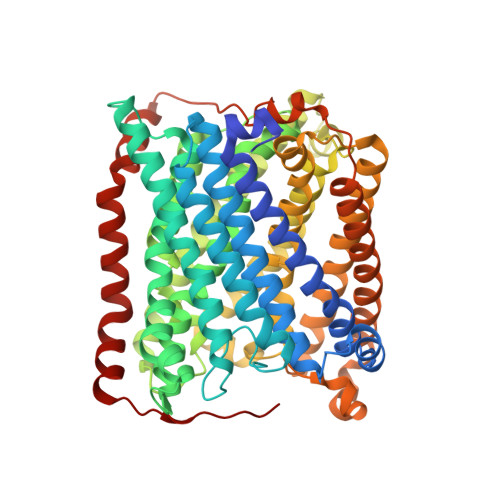
Cytochrome ba(3) oxidase is an integral membrane protein identified in the thermophilic bacterium Thermus thermophilus. The enzyme has now been expressed recombinantly and purified with a histidine tag. As such, it crystallizes under similar conditions and in the same space group (P4(3)2(1)2) as the native protein. A novel cryoprotection scheme is described here to obtain high-resolution diffraction from these crystals, which involves soaking in a mixture of glycerol and ethylene glycol under a layer of oil. The unit-cell parameters for these crystals are larger than the native protein, apparently deriving from increased ordering of the N-terminus and an internal loop (residues 495-500) in subunit I. Hence, compared with native cytochrome ba(3) oxidase, the recombinant His-tagged protein is accommodated in an expanded but equally well ordered lattice via an alternate set of specific intermolecular contacts. The structure was refined against data to 2.3 angstroms resolution to an R factor of 21.7% and an R(free) of 23.7%.
Organizational Affiliation:
Department of Molecular Biology, The Scripps Research Institute, MB8, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.