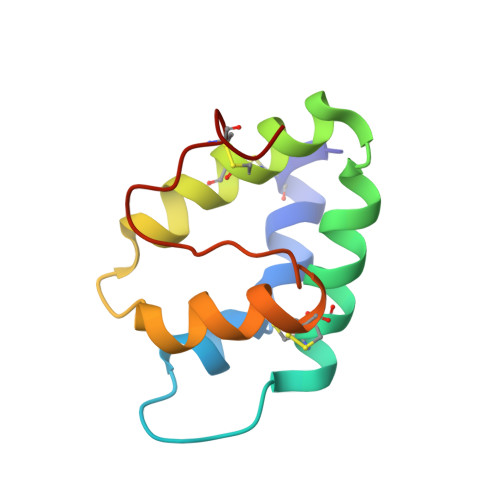
A novel lipid transfer protein from the dill Anethum graveolens L.: isolation, structure, heterologous expression, and functional characteristics.
Melnikova, D.N., Mineev, K.S., Finkina, E.I., Arseniev, A.S., Ovchinnikova, T.V.(2016) J Pept Sci 22: 59-66
- PubMed: 26680443
- DOI: https://doi.org/10.1002/psc.2840
- Primary Citation of Related Structures:
2N2Z - PubMed Abstract:
A novel lipid transfer protein, designated as Ag-LTP, was isolated from aerial parts of the dill Anethum graveolens L. Structural, antimicrobial, and lipid binding properties of the protein were studied. Complete amino acid sequence of Ag-LTP was determined. The protein has molecular mass of 9524.4 Da, consists of 93 amino acid residues including eight cysteines forming four disulfide bonds. The recombinant Ag-LTP was overexpressed in Escherichia coli and purified. NMR investigation shows that the Ag-LTP spatial structure contains four α-helices, forming the internal hydrophobic cavity, and a long C-terminal tail. The measured volume of the Ag-LTP hydrophobic cavity is equal to ~800 A(3), which is much larger than those of other plant LTP1s. Ag-LTP has weak antifungal activity and unpronounced lipid binding specificity but effectively binds plant hormone jasmonic acid. Our results afford further molecular insight into biological functions of LTP in plants.
- Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho-Maklaya str., 16/10, 117997, Moscow, Russia.
Organizational Affiliation: