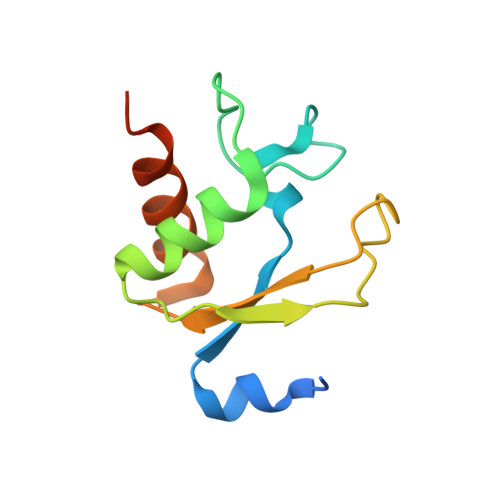
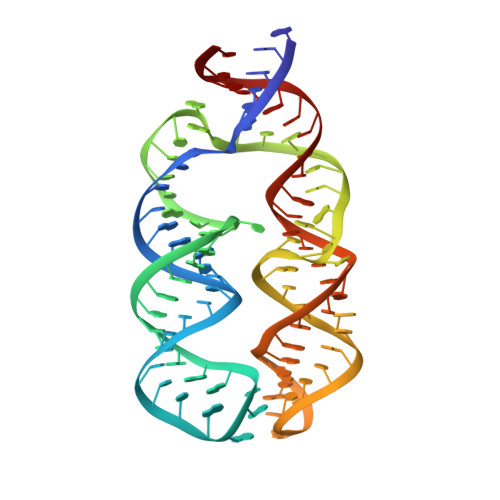
Structural insights into the assembly of the human and archaeal signal recognition particles.
Wild, K., Bange, G., Bozkurt, G., Segnitz, B., Hendricks, A., Sinning, I.(2010) Acta Crystallogr D Biol Crystallogr 66: 295-303
- PubMed: 20179341
- DOI: https://doi.org/10.1107/S0907444910000879
- Primary Citation of Related Structures:
3KTV, 3KTW - PubMed Abstract:
The signal recognition particle (SRP) is a conserved ribonucleoprotein (RNP) complex that co-translationally targets membrane and secretory proteins to membranes. The assembly of the particle depends on the proper folding of the SRP RNA, which in mammalia and archaea involves an induced-fit mechanism within helices 6 and 8 in the S domain of SRP. The two helices are juxtaposed and clamped together upon binding of the SRP19 protein to their apices. In the current assembly paradigm, archaeal SRP19 causes the asymmetric loop of helix 8 to bulge out and expose the binding platform for the key player SRP54. Based on a heterologous archaeal SRP19-human SRP RNA structure, mammalian SRP19 was thought not to be able to induce this change, thus explaining the different requirements of SRP19 for SRP54 recruitment. In contrast, the crystal structures of a crenarchaeal and the all-human SRP19-SRP RNA binary complexes presented here show that the asymmetric loop is bulged out in both binary complexes. Differences in SRP assembly between mammalia and archaea are therefore independent of SRP19 and are based on differences in SRP RNA itself. A new SRP-assembly scheme is presented.
Organizational Affiliation:
Heidelberg University Biochemistry Center (BZH), University of Heidelberg, INF328, D-69120 Heidelberg, Germany. klemens.wild@bzh.uni-heidelberg.de