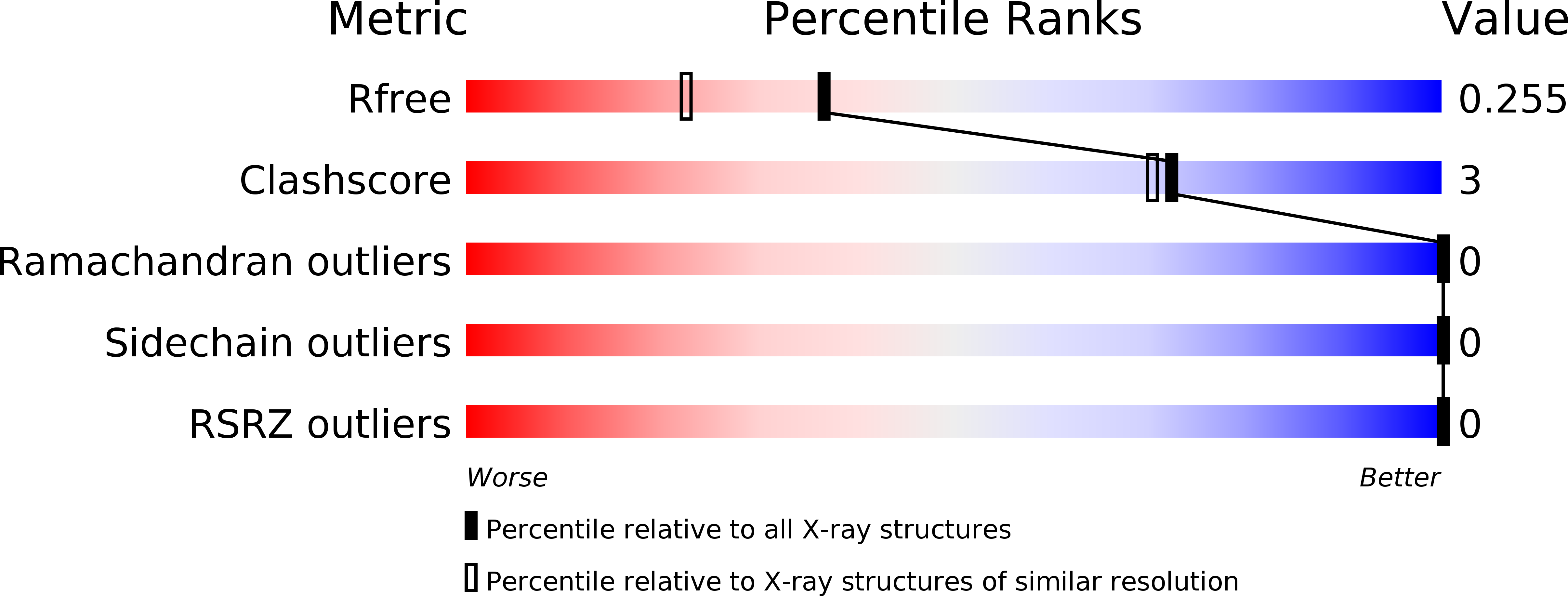
An Analysis of MIF Structural Features that Control Functional Activation of CD74.
Pantouris, G., Syed, M.A., Fan, C., Rajasekaran, D., Cho, T.Y., Rosenberg, E.M., Bucala, R., Bhandari, V., Lolis, E.J.(2015) Chem Biol 22: 1197-1205
- PubMed: 26364929
- DOI: https://doi.org/10.1016/j.chembiol.2015.08.006
- Primary Citation of Related Structures:
4P01, 4P0H, 4PKZ, 4PLU, 4TRF, 4TRU, 4XX7, 4XX8, 5BS9, 5BSC, 5BSI - PubMed Abstract:
For more than 15 years, the tautomerase active site of macrophage migration inhibitory factor (MIF) and its catalytic residue Pro1 have been being targeted for the development of therapeutics that block activation of its cell surface receptor, CD74. Neither the biological role of the MIF catalytic site nor the mechanistic details of CD74 activation are well understood. The inherently unstable structure of CD74 remains the biggest obstacle in structural studies with MIF for understanding the basis of CD74 activation. Using a novel approach, we elucidate the mechanistic details that control activation of CD74 by MIF surface residues and identify structural parameters of inhibitors that reduce CD74 biological activation. We also find that N-terminal mutants located deep in the catalytic site affect surface residues immediately outside the catalytic site, which are responsible for reduction of CD74 activation.
Organizational Affiliation:
Department of Pharmacology, Yale School of Medicine, New Haven, CT 06510, USA.