Expression to crystallization of diaminopimelate decarboxylase from the psychrophile Psychromonas ingrahamii
Peverelli, M.G., Newman, J., Panjikar, S., Perugini, M.A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Diaminopimelate decarboxylase | 427 | Psychromonas ingrahamii 37 | Mutation(s): 0 Gene Names: lysA, Ping_0041 EC: 4.1.1.20 | 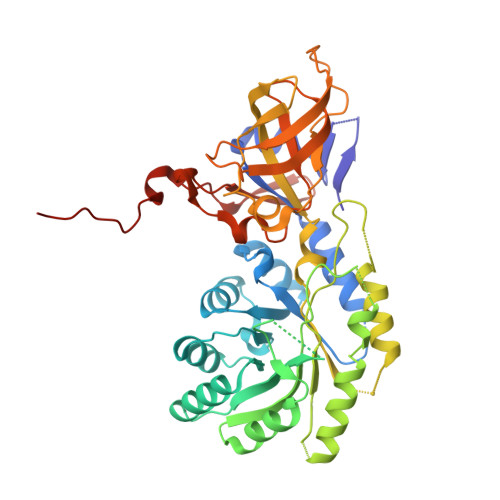 | |
UniProt | |||||
Find proteins for A1SR00 (Psychromonas ingrahamii (strain DSM 17664 / CCUG 51855 / 37)) Explore A1SR00 Go to UniProtKB: A1SR00 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A1SR00 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| LLP Query on LLP | BA [auth D], L [auth A], T [auth B], X [auth C] | (2S)-2-amino-6-[[3-hydroxy-2-methyl-5-(phosphonooxymethyl)pyridin-4-yl]methylideneamino]hexanoic acid C14 H22 N3 O7 P YQSOQJORMNSDJL-QFULYMJESA-N |  | ||
| SCN Query on SCN | E [auth A] F [auth A] G [auth A] H [auth A] M [auth B] | THIOCYANATE ION C N S ZMZDMBWJUHKJPS-UHFFFAOYSA-M |  | ||
| TME Query on TME | AA [auth D], K [auth A], Q [auth B], R [auth B], S [auth B] | PROPANE C3 H8 ATUOYWHBWRKTHZ-UHFFFAOYSA-N |  | ||
| K Query on K | I [auth A] J [auth A] O [auth B] P [auth B] W [auth C] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.96 | α = 90 |
| b = 103.13 | β = 94.77 |
| c = 93.59 | γ = 90 |
| Software Name | Purpose |
|---|---|
| Blu-Ice | data collection |
| MOSFLM | data reduction |
| SCALA | data scaling |
| SHELX | phasing |
| REFMAC | refinement |
| Coot | model building |
| XDS | data reduction |
| Auto-Rickshaw | phasing |