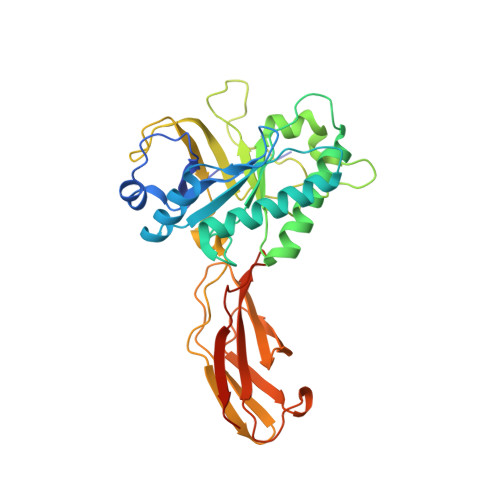
The crystal structure of Clostridium perfringens SleM, a muramidase involved in cortical hydrolysis during spore germination.
Al-Riyami, B., Ustok, F.I., Stott, K., Chirgadze, D.Y., Christie, G.(2016) Proteins 84: 1681-1689
- PubMed: 27488615
- DOI: https://doi.org/10.1002/prot.25112
- Primary Citation of Related Structures:
5JIP - PubMed Abstract:
Clostridium perfringens spores employ two peptidoglycan lysins to degrade the spore cortex during germination. SleC initiates cortex hydrolysis to generate cortical fragments that are degraded further by the muramidase SleM. Here, we present the crystal structure of the C. perfringens S40 SleM protein at 1.8 Å. SleM comprises an N-terminal catalytic domain that adopts an irregular α/β-barrel fold that is common to GH25 family lysozymes, plus a C-terminal fibronectin type III domain. The latter is involved in forming the SleM dimer that is evident in both the crystal structure and in solution. A truncated form of SleM that lacks the FnIII domain shows reduced activity against spore sacculi indicating that this domain may have a role in facilitating the position of substrate with respect to the enzyme's active site. Proteins 2016; 84:1681-1689. © 2016 Wiley Periodicals, Inc.
Organizational Affiliation:
Department of Chemical Engineering and Biotechnology, Institute of Biotechnology, University of Cambridge, Cambridge, United Kingdom.