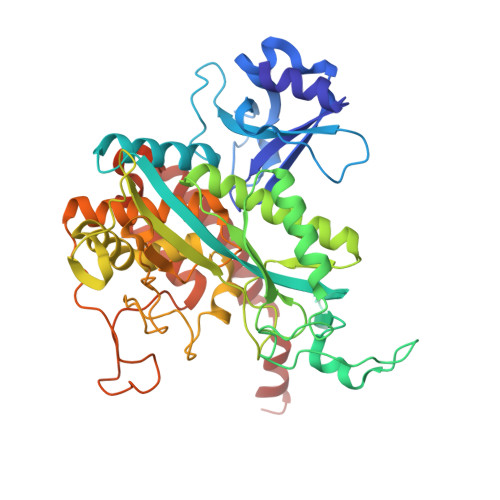
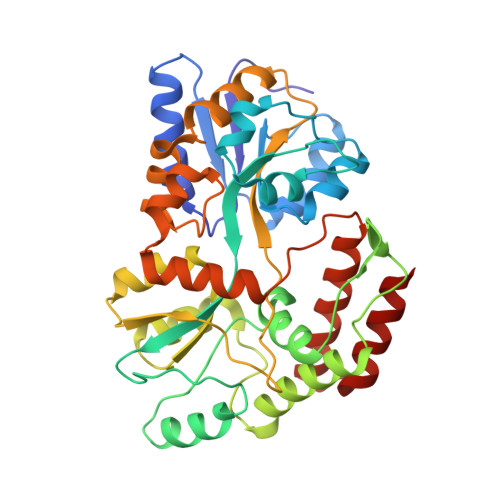
Fusion to a homo-oligomeric scaffold allows cryo-EM analysis of a small protein.
Coscia, F., Estrozi, L.F., Hans, F., Malet, H., Noirclerc-Savoye, M., Schoehn, G., Petosa, C.(2016) Sci Rep 6: 30909-30909
- PubMed: 27485862
- DOI: https://doi.org/10.1038/srep30909
- Primary Citation of Related Structures:
5LDF - PubMed Abstract:
Recent technical advances have revolutionized the field of cryo-electron microscopy (cryo-EM). However, most monomeric proteins remain too small (<100 kDa) for cryo-EM analysis. To overcome this limitation, we explored a strategy whereby a monomeric target protein is genetically fused to a homo-oligomeric scaffold protein and the junction optimized to allow the target to adopt the scaffold symmetry, thereby generating a chimeric particle suitable for cryo-EM. To demonstrate the concept, we fused maltose-binding protein (MBP), a 40 kDa monomer, to glutamine synthetase, a dodecamer formed by two hexameric rings. Chimeric constructs with different junction lengths were screened by biophysical analysis and negative-stain EM. The optimal construct yielded a cryo-EM reconstruction that revealed the MBP structure at sub-nanometre resolution. These findings illustrate the feasibility of using homo-oligomeric scaffolds to enable cryo-EM analysis of monomeric proteins, paving the way for applying this strategy to challenging structures resistant to crystallographic and NMR analysis.
Organizational Affiliation:
Institut de Biologie Structurale (IBS), Univ. Grenoble Alpes, CEA, CNRS, 38044 Grenoble, France.