ATP induced conformational changes facilitate E1-E2 disulfide bridging in the ubiquitin system
Misra, M., Schaefer, A., Kuhn, M., Pluska, L., Tessmer, I., Sommer, T., Schindelin, H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-conjugating enzyme E2-34 kDa | A [auth B], B [auth F], C [auth D], D [auth H] | 295 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: CDC34, DNA6, UBC3, YDR054C, D4211, YD9609.08C EC: 2.3.2.23 | 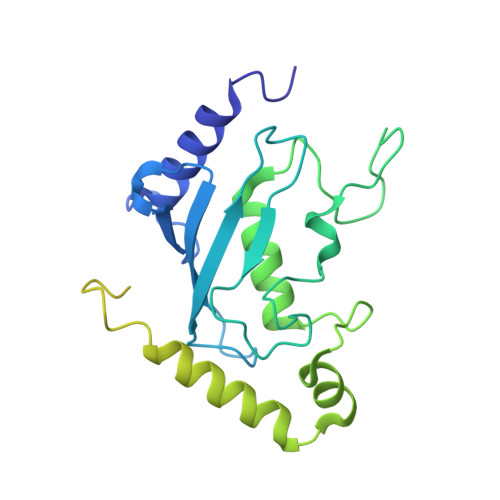 |
UniProt | |||||
Find proteins for P14682 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P14682 Go to UniProtKB: P14682 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14682 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-activating enzyme E1 1 | E [auth A], F [auth E], G [auth C], H [auth G] | 1,024 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: UBA1, YKL210W EC: 6.2.1.45 | 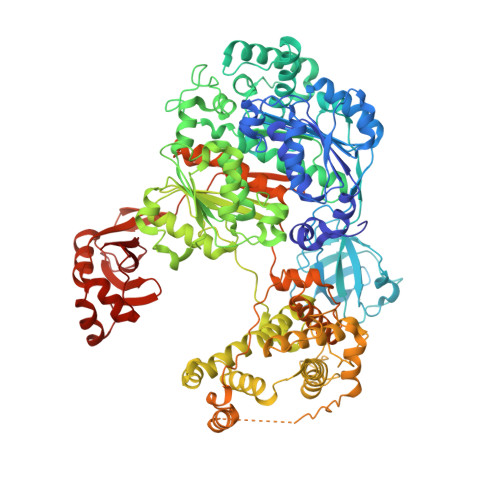 |
UniProt | |||||
Find proteins for P22515 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P22515 Go to UniProtKB: P22515 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P22515 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ATP (Subject of Investigation/LOI) Query on ATP | I [auth A], K [auth E], M [auth C], O [auth G] | ADENOSINE-5'-TRIPHOSPHATE C10 H16 N5 O13 P3 ZKHQWZAMYRWXGA-KQYNXXCUSA-N |  | ||
| MG Query on MG | J [auth A], L [auth E], N [auth C], P [auth G] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 75.636 | α = 90 |
| b = 152.408 | β = 90.448 |
| c = 252.567 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| PHASER | phasing |
| PARROT | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | SCHI 425-6/1 |