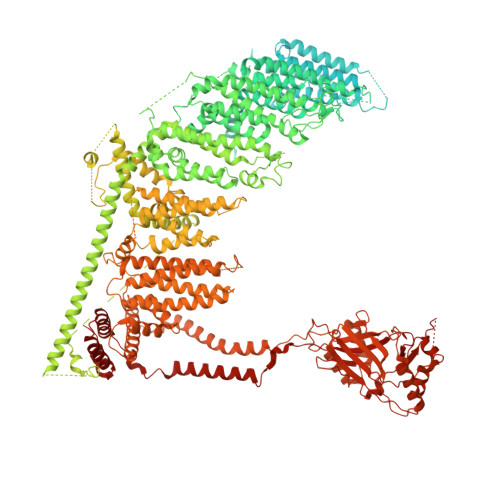
An intermediate open structure reveals the gating transition of the mechanically activated PIEZO1 channel.
Liu, S., Yang, X., Chen, X., Zhang, X., Jiang, J., Yuan, J., Liu, W., Wang, L., Zhou, H., Wu, K., Tian, B., Li, X., Xiao, B.(2025) Neuron 113: 590
- PubMed: 39719701
- DOI: https://doi.org/10.1016/j.neuron.2024.11.020
- Primary Citation of Related Structures:
8IXN, 8IXO - PubMed Abstract:
PIEZO1 is a mechanically activated cation channel that undergoes force-induced activation and inactivation. However, its distinct structural states remain undefined. Here, we employed an open-prone PIEZO1-S2472E mutant to capture an intermediate open structure. Compared with the curved and flattened structures of PIEZO1, the S2472E-Intermediate structure displays partially flattened blades, a downward and rotational motion of the top cap, and a spring-like compression of the linker connecting the cap to the pore-lining inner helix. These conformational changes open the cap gate and the hydrophobic transmembrane gate, whereas the intracellular lateral plug gate remains closed. The flattened structure of PIEZO1 with an up-state cap and closed cap gate might represent an inactivated state. Molecular dynamics (MD) simulations of ion conduction support the closed, intermediate open, and inactivated structural states. Mutagenesis and electrophysiological studies identified key domains and residues critical for the mechanical activation of PIEZO1. These studies collectively define the distinct structural states and gating transitions of PIEZO1.
- State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, Beijing Frontier Research Center of Biological Structure, Tsinghua University, Beijing 100084, China; MOE Key Laboratory of Protein Sciences, School of Life Sciences, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: