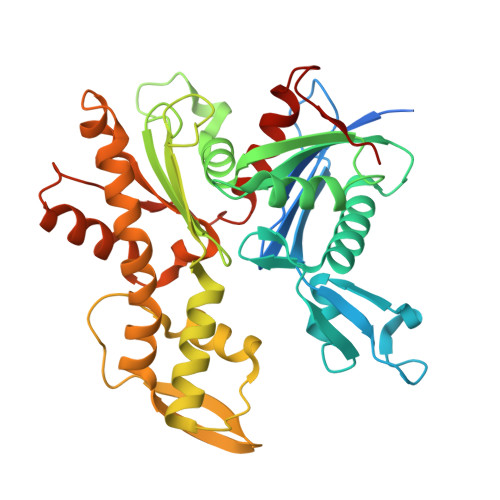
A family of bacterial actin homologs forms a three-stranded tubular structure.
Bergeron, J.R.C., Lale-Farjat, S.L.M., Lewicka, H.M., Parry, C., Kollman, J.M.(2025) Proc Natl Acad Sci U S A 122: e2500913122-e2500913122
- PubMed: 40073056
- DOI: https://doi.org/10.1073/pnas.2500913122
- Primary Citation of Related Structures:
8R2N - PubMed Abstract:
The cytoskeleton is crucial for cell organization and movement. In Eukaryotes, it largely consists of the protein actin, that forms a double-stranded linear filamentous structure in the presence of ATP and disassemble upon ATP hydrolysis. Bacteria also possess actin homologs, that drive fundamental cellular processes, including cell division, shape maintenance, and DNA segregation. Like eukaryotic actin, bacterial actins assemble into dynamic polymers upon ATP binding, however variation in interactions between strands gives rise to striking diversity of filament architectures. Here, we report a family of bacterial actins of unknown function, conserved among the Verrucomicrobiota phylum, which assembles into a unique tubular structure in the presence of ATP. A cryo-EM structure of the filaments reveals that it consists of three strands, unlike other described bacterial actin structures. This architecture provides further insights into the organization of actin-like filaments and has implications for understanding the diversity and evolution of the bacterial cytoskeleton.
- Randall Centre for Cell and Molecular Biophysics, King's College London, London SE1 1UL, United Kingdom.
Organizational Affiliation: