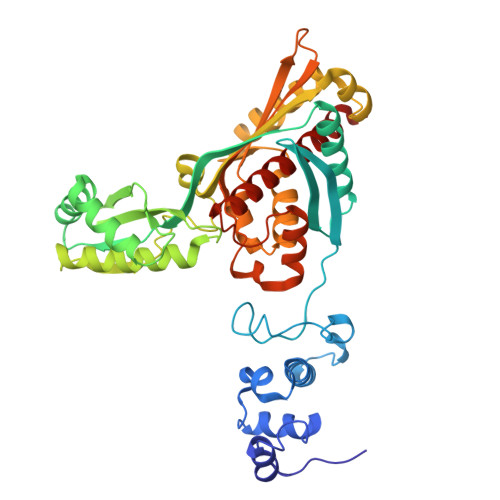
Cryo-EM structures of apo and atorvastatin-bound human 3-hydroxy-3-methylglutaryl-coenzyme A reductase.
Karuppasamy, M., van Rooyen, J.(2025) Acta Crystallogr F Struct Biol Commun 81: 118-122
- PubMed: 39976191
- DOI: https://doi.org/10.1107/S2053230X25001098
- Primary Citation of Related Structures:
8PKN, 8S6B - PubMed Abstract:
The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) regulates the level of cholesterol by catalysing the formation/production of mevalonate and has therefore become an important pharmaceutical target for coronary heart disease. Here, we report the cryo-EM structure of the catalytic part of the enzyme in the apo form and bound with its inhibitor atorvastatin, a commonly used drug in cardiovascular disease, at resolutions of 2.1 and 2.3 Å, respectively. In the cryo-EM maps, part of the N-domain corresponding to amino acids 439-487 is well ordered and could be modelled completely. Atorvastatin molecules were found to occupy all four active sites of the tetrameric complex, and the binding does not alter the conformation of the protein or the active site. The method described here exploits graphene oxide as an additional support and could be used as an alternative to elucidate the structures of pharmaceutical target compounds that are difficult to co-crystallize with human HMGR and for sparsely available samples in drug discovery.
- eBIC-for-Industry, Diamond Light Source, Harwell Science and Innovation Campus, Didcot OX11 0DE, United Kingdom.
Organizational Affiliation: