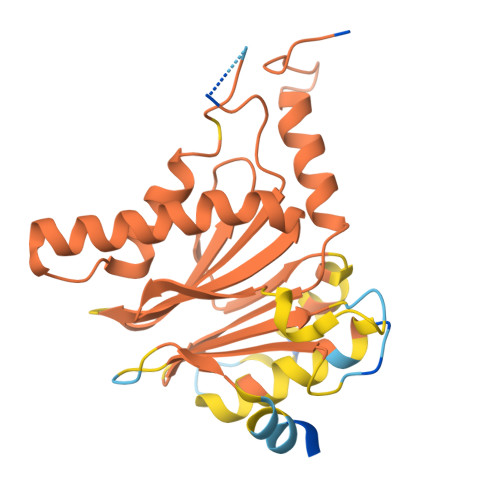
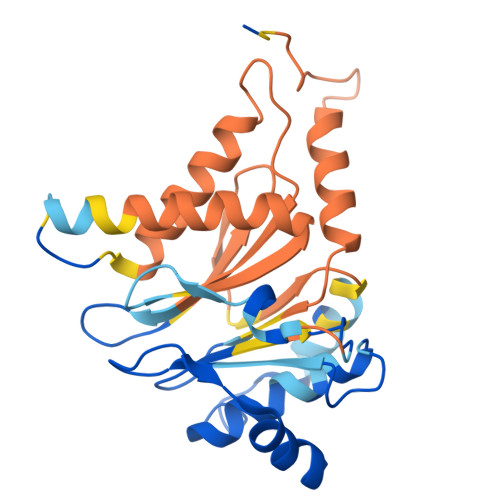
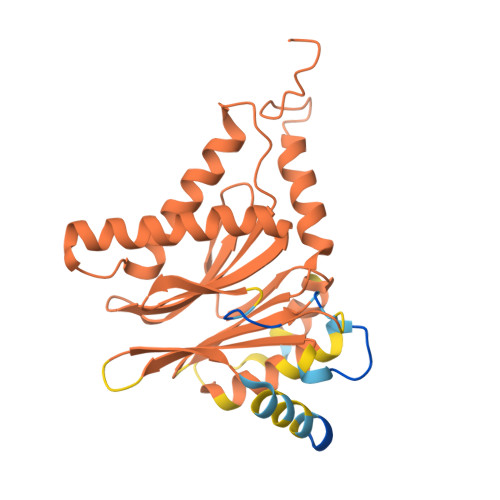
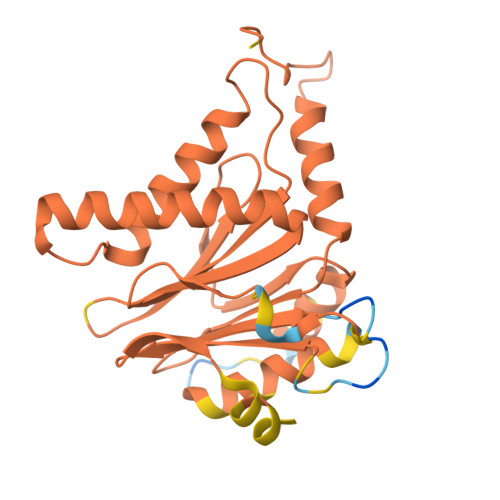
Optimization of alpha-amido boronic acids via cryo-electron microscopy analysis: Discovery of a novel highly selective immunoproteasome subunit LMP7 ( beta 5i)/LMP2 ( beta 1i) dual inhibitor.
Arai, Y., Shitama, H., Yamagishi, M., Ono, S., Kashima, A., Hiraizumi, M., Tsuda, N., Katayama, K., Tanaka, K., Koda, Y., Kato, S., Sakata, K., Nureki, O., Miyazaki, H.(2024) Bioorg Med Chem 109: 117790-117790
- PubMed: 38906067
- DOI: https://doi.org/10.1016/j.bmc.2024.117790
- Primary Citation of Related Structures:
8YPK, 8YSX, 8YVG, 8YVP - PubMed Abstract:
The immunoproteasome subunit LMP7 (β5i)/LMP2 (β1i) dual blockade has been reported to suppress B cell differentiation and activation, suggesting that the dual inhibition of LMP7/LMP2 is a promising approach for treating autoimmune diseases. In contrast, the inhibition of the constitutive proteasome subunit β5c correlates with cytotoxicity against non-immune cells. Therefore, LMP7/LMP2 dual inhibitors with high selectivity over β5c may be desirable for treating autoimmune diseases. In this study, we present the optimization and discovery of α-amido boronic acids using cryo-electron microscopy (cryo-EM). The exploitation of structural differences between the proteasome subunits led to the identification of a highly selective LMP7/LMP2 dual inhibitor 19. Molecular dynamics simulation based on cryo-EM structures of the proteasome subunits complexed with 19 explained the inhibitory activity profile. In mice immunized with 4-hydroxy-3-nitrophenylacetyl conjugated to ovalbumin, results indicate that 19 is orally bioavailable and shows promise as potential treatment for autoimmune diseases.
- Sohyaku. Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Electronic address: arai.yuuki@ma.mt-pharma.co.jp.
Organizational Affiliation: