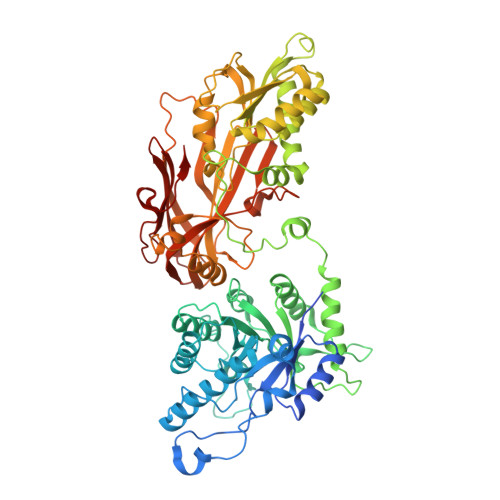
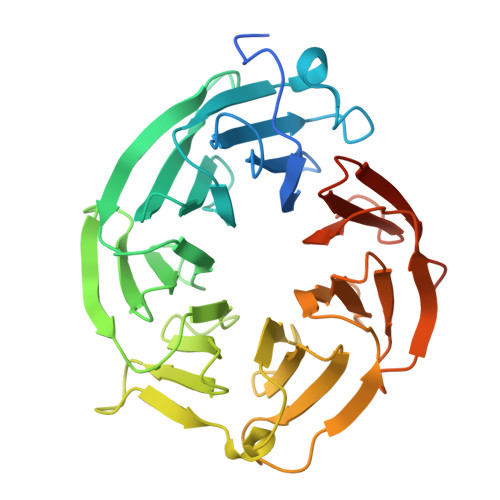
AMG 193, a Clinical Stage MTA-Cooperative PRMT5 Inhibitor, Drives Antitumor Activity Preclinically and in Patients with MTAP-Deleted Cancers.
Belmontes, B., Slemmons, K.K., Su, C., Liu, S., Policheni, A.N., Moriguchi, J., Tan, H., Xie, F., Aiello, D.A., Yang, Y., Lazaro, R., Aeffner, F., Rees, M.G., Ronan, M.M., Roth, J.A., Vestergaard, M., Cowland, S., Andersson, J., Sarvary, I., Chen, Q., Sharma, P., Lopez, P., Tamayo, N., Pettus, L.H., Ghimire-Rijal, S., Mukund, S., Allen, J.R., DeVoss, J., Coxon, A., Rodon, J., Ghiringhelli, F., Penel, N., Prenen, H., Glad, S., Chuang, C.H., Keyvanjah, K., Townsley, D.M., Butler, J.R., Bourbeau, M.P., Caenepeel, S., Hughes, P.E.(2025) Cancer Discov 15: 139-161
- PubMed: 39282709
- DOI: https://doi.org/10.1158/2159-8290.CD-24-0887
- Primary Citation of Related Structures:
9C10 - PubMed Abstract:
One of the most robust synthetic lethal interactions observed in multiple functional genomic screens has been dependency on PRMT5 in cancer cells with MTAP deletion. We report the discovery of the clinical stage MTA-cooperative PRMT5 inhibitor AMG 193, which preferentially binds PRMT5 in the presence of MTA and has potent biochemical and cellular activity in MTAP-deleted cells across multiple cancer lineages. In vitro, PRMT5 inhibition induces DNA damage, cell cycle arrest, and aberrant alternative mRNA splicing in MTAP-deleted cells. In human cell line and patient-derived xenograft models, AMG 193 induces robust antitumor activity and is well tolerated with no impact on normal hematopoietic cell lineages. AMG 193 synergizes with chemotherapies or the KRAS G12C inhibitor sotorasib in vitro, and combination treatment in vivo significantly inhibits tumor growth. AMG 193 is demonstrating promising clinical activity, including confirmed partial responses in patients with MTAP-deleted solid tumors from an ongoing phase 1/2 study.
- Amgen Inc., Thousand Oaks, CA, United States.
Organizational Affiliation: