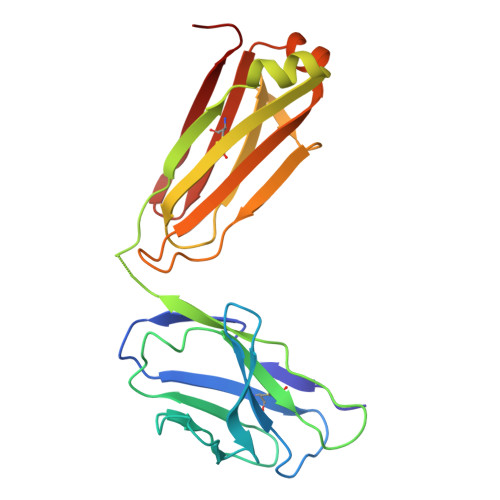
An alpha IIb beta 3 ligand-mimetic murine monoclonal antibody that produces platelet activation by engaging the Fc gamma IIa receptor.
Wang, J., Buitrago, L., Wang, L., Li, J., Walz, T., Coller, B.S.(2025) Blood Adv 9: 3518-3529
- PubMed: 40101235
- DOI: https://doi.org/10.1182/bloodadvances.2024015368
- Primary Citation of Related Structures:
9DAO, 9DAX - PubMed Abstract:
To produce a murine monoclonal antibody that binds to αIIbβ3 and inhibits clot retraction (CR) we immunized mice with human platelets and tested hybridoma supernatants for their ability to bind to αIIbβ3 and inhibit CR. The IgG1 mAb R6H8 completely inhibited CR at 20 µg/ml. Paradoxically, 5 µg/ml R6H8 initiated platelet aggregation and induced P-selectin expression, fibrinogen binding, and PAC-1 binding. At 20 µg/ml, however, R6H8 completely inhibited aggregation induced by peptide SFLLRN (25 µg/ml; T6) and T6-induced fibrinogen and PAC-1 binding to platelets. Platelet aggregation induced by R6H8 was inhibited by mAb IV.3, which blocks the FcγIIa receptor (FcγRIIa), and the Fab fragment of R6H8 did not induce platelet aggregation, suggesting that R6H8 binds to both αIIbβ3 and FcγRIIa. Cryo-EM analysis of the R6H8 Fab-αIIbβ3 complex revealed that R6H8: 1) Binds to the αIIbβ3 RGD binding pocket via an RYD sequence in its heavy chain CDR3, 2) Interacts with β3 Asp126, producing a reorientation of Asp126 and loss of the ADMIDAS Ca2+, and 3) Initiates swing-out of the β3 hybrid domain. We conclude that R6H8 is an αIIbβ3 ligand-mimetic mAb that activates platelets via FcγRIIa at low concentrations and potently inhibits platelet aggregation and clot retraction at high concentrations. R6H8 simulates the actions of a number of pathological antibodies, including platelet-activating antibodies developed after therapy with glycoprotein IIb/IIIa (αIIbβ3) inhibitors and platelet-activating antibodies in heparin-induced thrombocytopenia and vaccine-induced immune thrombotic thrombocytopenia. As such, it may be a valuable reagent for better understanding these disorders and identifying potential therapies.
- The Rockefeller University, NEW YORK, New York, United States.
Organizational Affiliation: