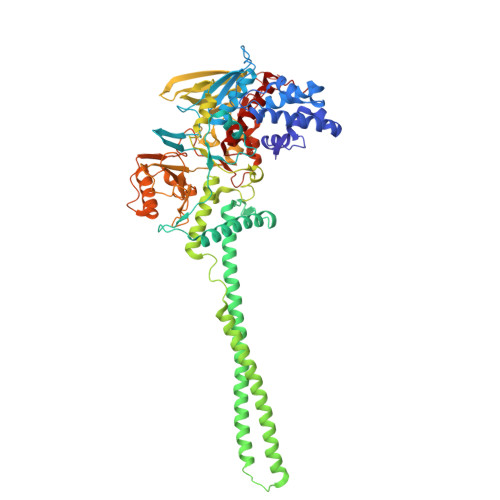
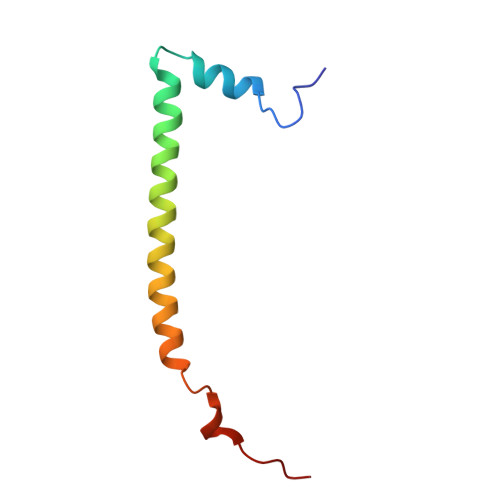
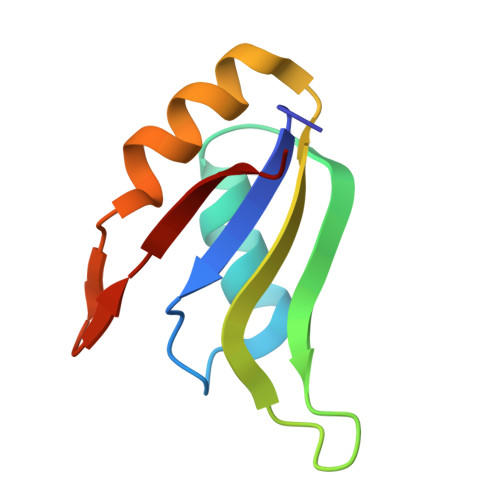
CoREST Complex Inhibition Alters RNA Splicing to Promote Neoantigen Expression and Enhance Tumor Immunity.
Fisher, R.J., Park, K., Lee, K., Pinjusic, K., Vanasse, A., Ennis, C.S., Ficcaro, S., Marto, J., Stransky, S., Duke-Cohan, J., Geethadevi, A., Raabe, E., Sidoli, S., Hicks, C.W., Keskin, D.B., Wu, C.J., Cole, P.A., Alani, R.M.(2024) bioRxiv
- PubMed: 39713349
- DOI: https://doi.org/10.1101/2024.12.12.627852
- Primary Citation of Related Structures:
9DWU - PubMed Abstract:
Epigenetic complexes tightly regulate gene expression and colocalize with RNA splicing machinery; however, the consequences of these interactions are uncertain. Here, we identify unique interactions of the CoREST repressor complex with RNA splicing factors and their functional consequences in tumorigenesis. Using mass spectrometry, in vivo binding assays, and cryo-EM we find that CoREST complex-splicing factor interactions are direct and perturbed by the CoREST complex inhibitor, corin, leading to extensive changes in RNA splicing in melanoma and other malignancies. Using predictive machine learning models and MHC IP-MS, we identify thousands of corin-induced neopeptides derived from unannotated splice sites which generate immunogenic splice-neoantigens. Furthermore, corin reactivates the response to immune checkpoint blockade and promotes dramatic expansion of cytotoxic T cells in an immune cold melanoma model. CoREST complex inhibition thus represents a unique therapeutic opportunity in cancer which creates tumor-associated neoantigens that enhance the immunogenicity of current therapeutics. We identify a novel role of the CoREST transcriptional repressor complex in regulating pre-mRNA splicing and find that the small molecule inhibitor, corin, promotes alternative splicing events in cancer leading to neoantigen expression and T cell-mediated immunity. This represents a potential approach to promote immunoreactive neoantigen expression in immune-cold tumors.