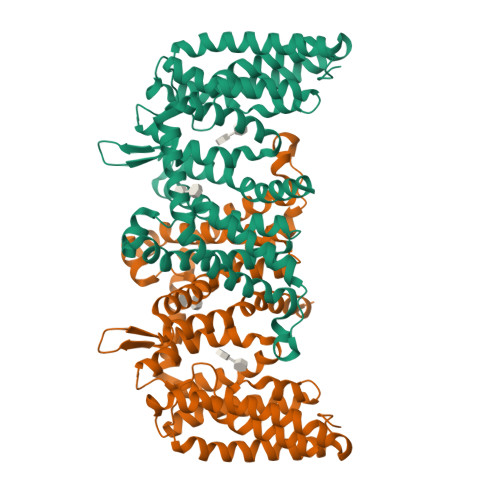
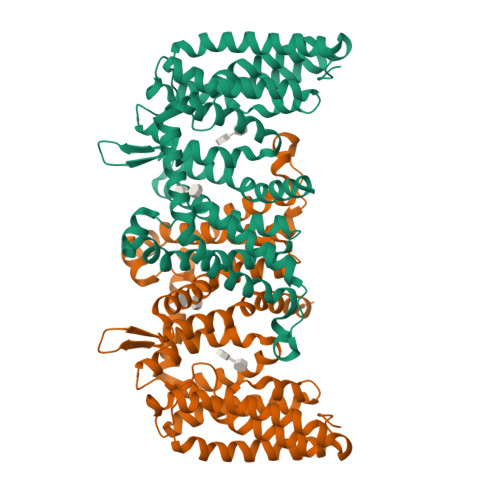
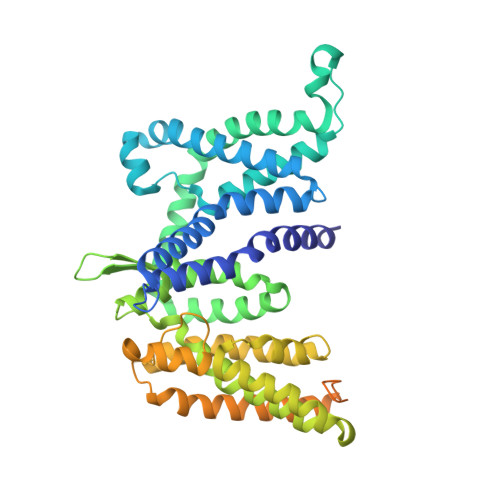
Cryo-EM structure of a phosphotransferase system glucose transporter stalled in an intermediate conformation.
Roth, P., Fotiadis, D.(2025) J Struct Biol X 11: 100124-100124
- PubMed: 40124667
- DOI: https://doi.org/10.1016/j.yjsbx.2025.100124
- Primary Citation of Related Structures:
9HNP - PubMed Abstract:
The phosphotransferase system glucose-specific transporter IICB Glc serves as a central nutrient uptake system in bacteria. It transports glucose across the plasma membrane via the IIC Glc domain and phosphorylates the substrate within the cell to produce the glycolytic intermediate, glucose-6-phosphate, through the IIB Glc domain. Furthermore, IIC Glc consists of a transport (TD) and a scaffold domain, with the latter being involved in dimer formation. Transport is mediated by an elevator-type mechanism within the IIC Glc domain, where the substrate binds to the mobile TD. This domain undergoes a large-scale rigid-body movement relative to the static scaffold domain, translocating glucose across the membrane. Structures of elevator-type transporters are typically captured in either inward- or outward-facing conformations. Intermediate states remain elusive, awaiting structural determination and mechanistic interpretation. Here, we present a single-particle cryo-EM structure of purified, n -dodecyl-β-D-maltopyranoside-solubilized IICB Glc from Escherichia coli . While the IIB Glc protein domain is flexible remaining unresolved, the dimeric IIC Glc transporter is found trapped in a hitherto unobserved intermediate conformational state. Specifically, the TD is located halfway between inward- and outward-facing states. Structural analysis revealed a specific n -dodecyl-β-D-maltopyranoside molecule bound to the glucose binding site. The sliding of the TD is potentially impeded halfway due to the bulky nature of the ligand and a shift of the thin gate, thereby stalling the transporter. In conclusion, this study presents a novel conformational state of IIC Glc , and provides new structural and mechanistic insights into a potential stalling mechanism, paving the way for the rational design of transport inhibitors targeting this critical bacterial metabolic process.
Organizational Affiliation:
Institute of Biochemistry and Molecular Medicine, Medical Faculty, University of Bern, Bern, Switzerland.