Structural basis of lipopolysaccharide translocon assembly mediated by the small lipoprotein LptM
Miyazaki, R., Kimoto, M., Kohga, H., Tsukazaki, T.(2025) Cell Rep 44
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2025) Cell Rep 44
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| LPS-assembly protein LptD | 760 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: lptD, imp, ostA, yabG, b0054, JW0053 | 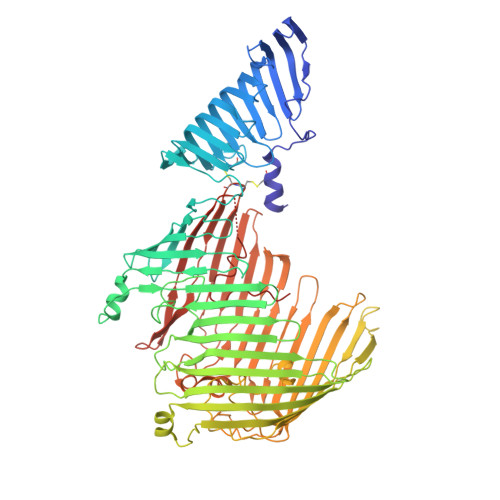 | |
UniProt | |||||
Find proteins for P31554 (Escherichia coli (strain K12)) Explore P31554 Go to UniProtKB: P31554 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31554 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| LPS-assembly lipoprotein LptE | 175 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: lptE, rlpB, b0641, JW0636 | 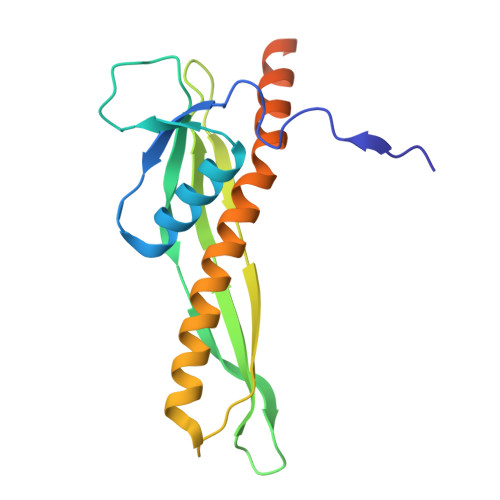 | |
UniProt | |||||
Find proteins for P0ADC1 (Escherichia coli (strain K12)) Explore P0ADC1 Go to UniProtKB: P0ADC1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0ADC1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uncharacterized lipoprotein YifL | 60 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: yifL, b4558, JW3781 | 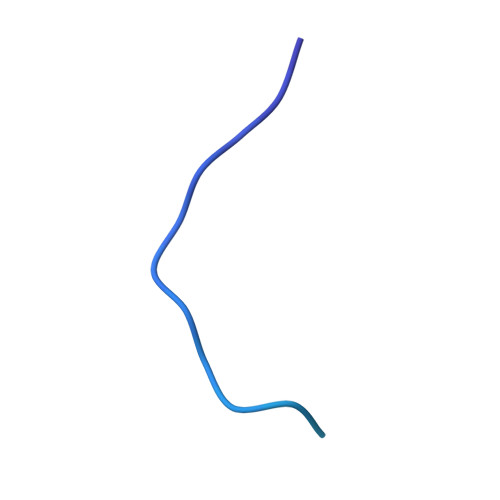 | |
UniProt | |||||
Find proteins for P0ADN6 (Escherichia coli (strain K12)) Explore P0ADN6 Go to UniProtKB: P0ADN6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0ADN6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Japan Society for the Promotion of Science (JSPS) | Japan | -- |